oncology
2010 American Society of Clinical Oncology (ASCO) Annual Meeting
New Data from Randomized Phase III Trials Do Not Alter Standard Therapy for Brain Cancer in the Elderly
Chicago – In glioblastoma, the gold standard of an initial combination of radiotherapy and chemotherapy followed by adjuvant chemotherapy does not appear to have been altered by new results from two phase III studies conducted in elderly patients. Presented as late breaker data at this year’s ASCO meeting, the studies are part of an ongoing effort to identify the best modalities for improved outcomes in the context of acceptable tolerability. Both studies were conducted in newly diagnosed elderly patients but ultimately served as a platform of debate about the definition of elderly and the role of age as a discriminator for treatment independent of performance status. The results of the studies underscore the complex balance between median overall survival benefit and quality of life. Relative to age, discriminators such as O-6-methylguanine DNA methyltransferase (MGMT) promoter methylation status may be more useful for selecting intensity of therapy in an effort to extend survival at an acceptable level of tolerability.
Phase III Trials Test Modified Glioblastoma Therapy in the Elderly
Two new phase III studies conducted in elderly patients with newly diagnosed glioblastoma have reinforced the standard of concomitant radiotherapy (RT) and temozolomide (TMZ) followed by adjuvant TMZ in patients with good performance status. Although the studies addressed slightly different questions about how best to manage newly diagnosed glioblastoma in an elderly population, the conclusion drawn by the invited ASCO discussant Dr. Stuart Grossman, Professor of Medicine, Oncology, and Neurological Surgery, Johns Hopkins Medical School, Baltimore, Maryland, was that age is not a useful isolated criterion for treatment selection. A referee for the two latebreaker presentations, he concluded that the data do not alter the current standard first-line therapy for newly diagnosed glioblastoma.
“Radiation plus temozolomide followed by six months of adjuvant temozolomide is certainly the standard treatment and is probably the optimal therapy for fit glioblastoma patients of any age.”
Based on the most recent and previously published data, “radiation plus TMZ followed by six months of adjuvant TMZ is certainly the standard treatment and is probably the optimal therapy for fit glioblastoma patients of any age,” reported Dr. Grossman. “Chronological age does not necessarily reflect relative fitness, and it is important that patients be given a therapy most likely to extend survival in the absence of clear contraindications.”
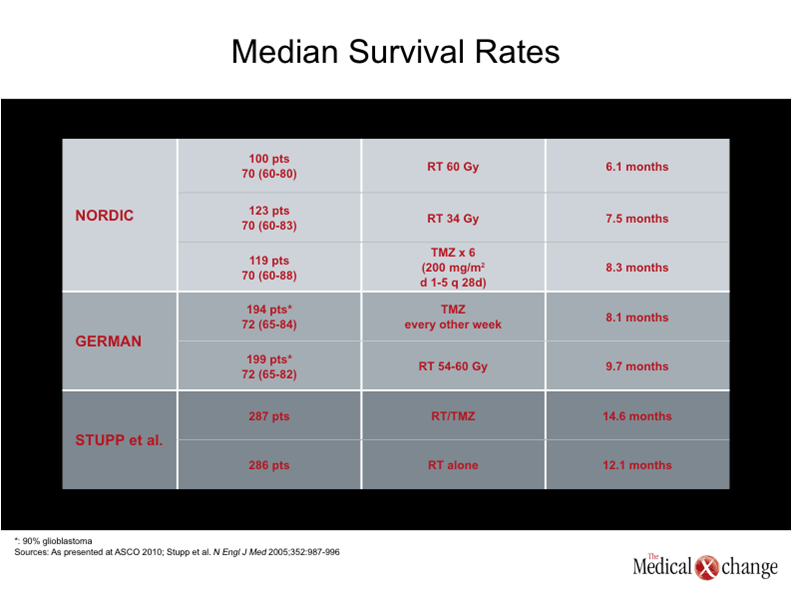
The standard of radiation plus TMZ was established by a multicenter study published five years ago (Stupp et al. N Engl J Med 2005;352:987-996). Patients were randomized to receive RT alone (5 days a week for six weeks for a total dose of 60 Gy) or the same dose of RT with concomitant TMZ (75 mg/m2 seven days per week during the course of RT) followed by adjunctive TMZ (150 to 200 mg/m2 for five days for each of six 28-day cycles). After a median follow-up of 28 months, the median survival was modestly improved with concomitant and adjuvant TMZ (14.6 vs. 12.1 months; p<0.001), but the long-term survival was far superior. In the original report, 26.5% of those receiving TMZ vs. 10.4% of those receiving RT alone were alive at the end of two years.
This background is important for understanding the two most recent studies, because the effort to adjust the regimens for elderly patients did not yield median survival rates of the same magnitude as the trial credited with establishing the treatment standard in newly diagnosed glioblastoma. In one of the late breaker phase III studies presented at ASCO, elderly patients—defined as age 65 years or older—with newly diagnosed glioblastoma or anaplastic astrocytoma were randomized to either RT in the same dosage range used by Stupp et al., or to TMZ in a one week on, one week off schedule of 100 mg/m2 (dose modifications allowed). The plan was to employ the opposite therapy at the time of disease progression, producing a better tolerated and more convenient treatment strategy.
However, non-inferiority for TMZ was not shown, and few patients in either arm were ever crossed over to the opposite therapy, according to Dr. Wolfgang Wick, Department of Neurooncology, University of Heidelberg, Germany. Anticipating the same comments made by Dr. Grossman in assessing these results, Dr. Wick reported that “RT cannot be safely deferred in elderly patients by initiating treatment with TMZ alone.”
New Study Supports Lower Radiation Doses in the Elderly
Explaining the design of this German phase III study, called NOA-08, Dr. Wick reported that 143 patients were randomized, most of whom (approximately 90%) had glioblastoma. A Karnofsky score greater than 60 was required for entry and the median Karnofsky score was 80. The median age was 71. The primary endpoint was median survival, which was 293 days (9.7 months) in the RT group and 245 days (8.1 months) in the TMZ group. This difference did not permit TMZ to meet the predefined boundary of non-inferiority (Graph 1).
In the second phase III study, conducted by the Nordic Brain Tumor Study Group, 291 newly diagnosed “elderly” patients, defined as 60 years of age or older, were randomized to one of three arms: a standard RT with a total dose of 60 Gy delivered over six weeks, a reduced RT dose of 34 Gy over the same period of time, or TMZ administered in a dose of 200 mg/m2 for five consecutive days of each six 28-day cycles. Although this study, like the German study, employed the established radiation dose in one of the study arms, both the German and the Nordic studies deviated from the conventional TMZ regimens.
“For patients between the ages of 60 and 70, there were no differences between the treatment arms, but for older patients TMZ offered longer survival than RT at the higher dose and in no case was the higher dose of RT superior to the lower dose.”
The median survival overall was 8.3 months for TMZ, 7.5 months for 34 Gy dose of RT, and 6.0 months for the 60 Gy dose of RT. These differences did not reach statistical significance but the median survival rates in patients over the age of 70 were 9.0 months, 7.1 months, and 5.2 months, respectively, and these were significantly different (p<0.001 for TMZ vs. RT and p=0.02 for low dose RT vs. high dose RT).
“For patients between the ages of 60 and 70, there were no differences between the treatment arms, but for older patients TMZ offered longer survival than RT at the higher dose and in no case was the higher dose of RT superior to the lower dose,” reported Dr. Annika Malmström, Department of Oncology, Linköping University Hospital, Sweden, who concluded that 60 Gy RT “should be avoided in elderly patients.”
However, the term “elderly” was challenged by Dr. Grossman, who considers a set age limit arbitrary. Regardless of the definition for elderly, Dr. Grossman suggested that fitness level rather than age should be used to determine therapy. He noted that the median survival rates in these studies, which ranged from 6.1 to 9.7 months, were lower than the survival associated with RT + TMZ followed by adjunctive TMZ, which exceeded 12 months. For that reason, he concluded that this standard be retained in any patient with an acceptable performance status.
“We are going to have to think of frailty and fitness as discriminators rather than age, particularly as the evidence suggests that the gold standard as it is currently defined provides a substantial advantage for fit patients over the alternatives studied so far.”
One reason that it will be important to look at methods other than age for stratifying patients is that the average age at the time of diagnosis of glioblastoma is in the range of 60 to 65 years, which was “elderly” as defined in the Nordic study. According to Dr. Grossman, such prognostic indicators as MGMT promoter methylation status are far more promising for patient stratification. He cited one recent study in which positive MGMT promoter methylation status was associated with a substantial increase in median survival relative to negative status (16.2 vs. 8.7 months; p=0.002) (Gerstner et al. Neurology 2009;73:1509-1510).
“We are going to have to think of frailty and fitness as discriminators rather than age, particularly as the evidence suggests that the gold standard as it is currently defined provides a substantial advantage for fit patients over the alternatives studied so far,” Dr. Grossman reported.