oncology
The 35th European Society for Medical Oncology (ESMO) Congress
Latest Advances in the Prevention and Control of Chemotherapy-induced Nausea and Vomiting
Milan – Dramatic progress has occurred over the past three decades in the management of chemotherapy-induced nausea and vomiting (CINV). Control rates have increased from 0-10% in 1980 to 75-85% in 2010. Still, many cancer patients endure unnecessary bouts of emesis due to inadequate prophylactic treatment. Nausea complicates an even greater proportion of chemotherapy cycles, as less progress has occurred in controlling that adverse effect as compared with vomiting. With currently available medications, clinicians have the means to eliminate CINV, which should be the goal for every cancer patient who requires chemotherapy. Adherence to clinical guidelines offers the potential to achieve the goal of preventing nausea and vomiting in every cancer patient who receives chemotherapy. Recent updates of guidelines have increased emphasis on use of NK1 antagonists as an essential component of strategies to prevent and control CINV in cancer patients.
Eliminating CINV is Possible
Clinicians should set higher goals for controlling chemotherapy-induced nausea and vomiting (CINV), said Richard Gralla, MD, Professor of Medicine, North Shore-LIJ, Hofstra University, Lake Success, NY, at the ESMO Congress this year. “There is no such thing as an acceptable level of CINV. We have the means to eliminate it and that should be the goal for every patient who receives highly or moderately emetogenic chemotherapy,” explained Dr. Gralla. Reaching that goal begins with bringing clinical perceptions in line with patient realities.
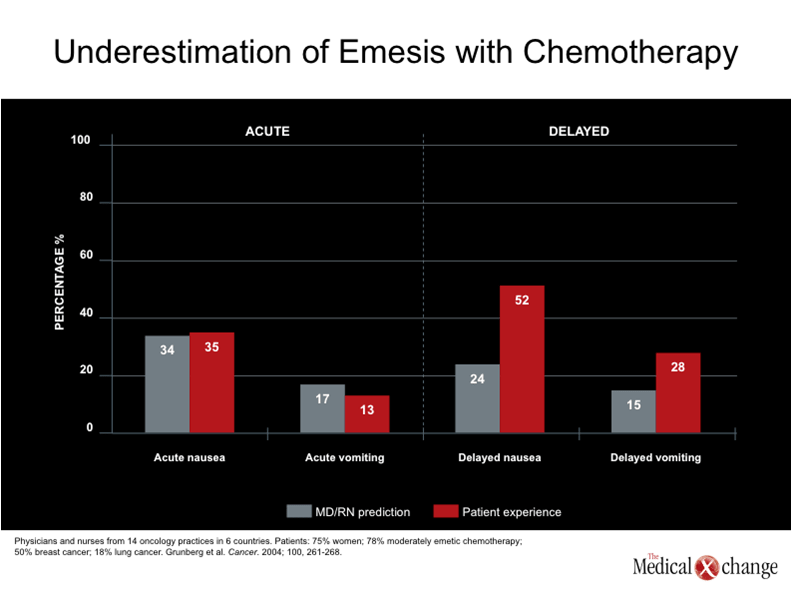
One telling study compared physician and nurse predictions about the emetogenicity of chemotherapy with patient reported experiences (Grunberg et al. Cancer, 2004; 100(10): 2261-8). Perception and reality were in accord for acute nausea and vomiting. However, patient-reported problems with delayed nausea and vomiting were twice as high as perceived by clinicians (52% vs. 24% for nausea and 28% vs. 15% for vomiting) (Table 1).
Optimal antiemetic therapy involves use of the best dose of each agent and recognition of and adherence to the most effective and convenient schedules.
A second necessary step towards eliminating CINV is the optimal use of available antiemetic agents, particularly from the three major classes: corticosteroids, serotonin 5-HT3 antagonists, and substance P/neurokinin 1 (NK1) receptor antagonists.
“Optimal antiemetic therapy involves use of the best dose of each agent and recognition of and adherence to the most effective and convenient schedules,” explained Dr. Gralla.
Continuing Role for Corticosteroids
After decades of use, corticosteroids remain a key component of effective antiemetic therapy, said Dr. Gralla. The value of the class was illustrated in a meta-analysis published more than a decade ago. The analysis examined randomized clinical trials comparing control of acute emesis with 5-HT3 antagonists alone, or in combination with dexamethasone. The results showed consistently better outcomes with the combination across all of the trials, resulting in a highly significant (P<0.00001) 53% overall reduction in the odds ratio in favour of using 5-HT3 antagonists with dexamethasone (Jantunen IT et al. Eur J Cancer. 1997;33:66-74).
More recently, the effect of dexamethasone on delayed emesis was examined in a placebo-controlled randomized clinical trial that compared the 5-HT3 antagonist palonosetron alone or in combination with the corticosteroid in women with metastatic breast cancer treated with moderately emetogenic chemotherapy regimens (Aapro M. et al. Ann Oncol.2010;21:1083-8). All patients received both agents on day 1 and then were randomized to receive a reduced dose of dexamethasone or placebo on days 2 and 3.
“The results showed no significant difference in the rate of complete response or control of acute and delayed emesis,” Dr. Gralla explained. “However, the dexamethasone arm exhibited a trend toward better control of delayed nausea.”
Targeting the 5-HT3 Receptor
Clinicians have multiple options among the 5-HT3 antagonists, which have receptor binding affinities that range from 7.6 pKi (dolasetron) to 10.4 pKi (palonosetron). Single-agent comparison trials suggested that the newer agent palonosetron offers superior control of CINV, said Dr. Gralla. However, the relative efficacy of 5-HT3 antagonists in combination with dexamethasone remained unclear until the recent publication of findings from a large randomized clinical trial (Gralla, R, Raftopoulos, H. Lancet Oncol. 2009;10:115-24).
The trial involved 1,114 cancer patients receiving cisplatin or doxorubicin (or epirubicin)/ cyclophosphamide chemotherapy. They were randomized to antiemetic therapy with palonosetron/dexamethasone or granisetron/dexamethasone. The primary endpoints were complete response (no emesis or rescue medication) during the acute (0-24 hours) and delayed (24-120 hours) phases.
Both regimens had complete response rates of about 75% during the acute phase, but the palonosetron regimen showed a significantly higher rate of complete response during the delayed phase (56.8% vs. 44.5%, P=0.0001) in overall control (51.5% vs. 40.4%, P=0.0001).
Enhanced Control with NK1 Antagonism
The addition of NK1 antagonists to the antiemetic armamentarium has given clinicians a powerful new tool to control CINV and improve cancer patients’ quality of life, Dr. Gralla said. PET imaging studies have demonstrated almost 100% occupancy of NK1 receptors after a single dose of aprepitant (Hargreaves R. J Clin Psychiatry. 2002;63(Suppl 11):18-24).
Aprepitant has demonstrated substantial antiemetic efficacy and is recommended as a component of standard prophylaxis in clinical guidelines, Dr. Gralla continued. The value of the agent was demonstrated in a phase III clinical trial that compared ondansetron/dexamethasone antiemetic therapy with or without the addition of aprepitant in 1,043 cancer patients receiving cisplatin-based chemotherapy (Hesketh PJ et al. J Clin Oncol. 2003;21:4112-9). The results showed significant improvement in complete response rates for acute (86% vs. 73%, P<0.001) and delayed (72% vs. 51%, P<0.001) emesis.
“A 50% improvement in control of delayed emesis is particularly impressive,” Dr. Gralla confirmed.
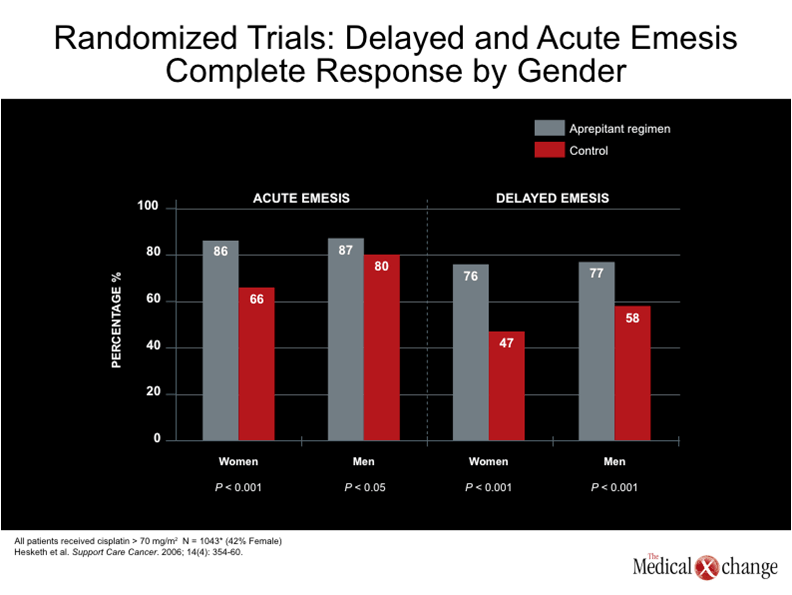
Further, aprepitant has proven to be particularly beneficial to female cancer patients, who tend to be more susceptible to the emetogenic effects of chemotherapy and less responsive to conventional antiemetic therapy. An analysis of randomized clinical trials showed that men and women alike achieved similar control of acute and delayed emesis with aprepitant containing regimens, whereas women had substantially lower complete response rates with control regimens (Hesketh PJ et al. Support Care Cancer. 2006;14:354-60) (Table 2).
Evidence-Based Therapy
The quest for complete elimination of CINV recently led to a clinical evaluation of the combination of palonosetron, dexamethasone, and aprepitant in patients treated primarily with anthracycline-based chemotherapy (Grunberg SM et al. Support Care Cancer. 2009;17:589-594). The results showed that no patient had acute episodes of emesis, and the rate of delayed emesis was 5%. Corresponding results for nausea were 78% and 68%, respectively. Overall, 95% of patients had no episodes of emesis over five days of follow-up, and 66% reported no nausea.
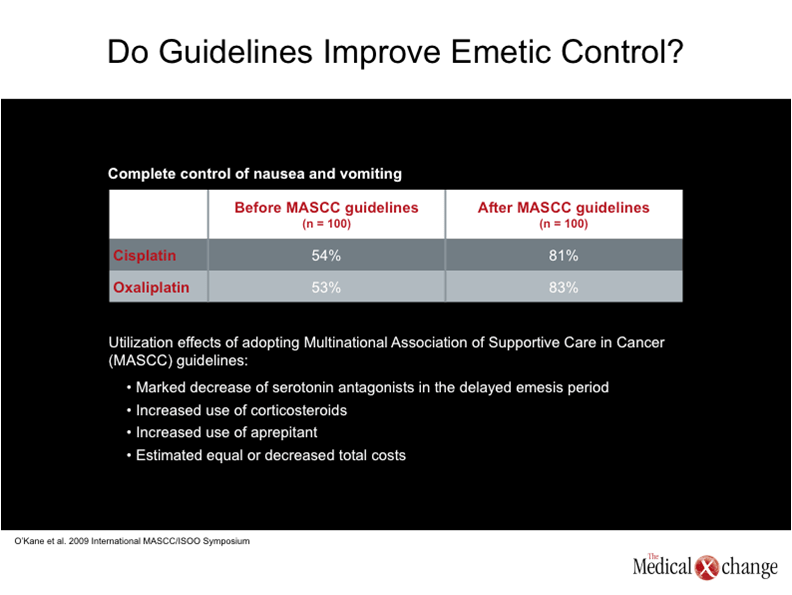
Progress in the management of CINV has attracted considerable attention in various clinical guidelines, Dr. Gralla said. The value of adherence to clinical guidelines emerged clearly from a recent comparison of clinician practices before and after implementation of the Multinational Association of Supportive Care in Cancer recommendations for antiemetic therapy (Proc MASCC. 2009). The latest version of the guidelines emphasizes the decreased use of 5-HT3 antagonists in the management of delayed emesis; the increased use of corticosteroids; and the increased use of aprepitant.
A survey of 100 clinicians showed that the rate of complete control of nausea and vomiting in patients receiving cisplatin- or oxaliplatin-based regimens was 53-54% before implementation of the MASCC guidelines. The rate of complete control increased to greater than 80% after clinicians began following the MASCC recommendations (Table 3).
An economic analysis showed that adherence to the guidelines was associated with equivalent or perhaps even lower costs compared with practices before the guidelines were implemented.
“The issue is whether we are going to practice evidence-based medicine or not. We have the means to eliminate CINV. Why would we want our patients to risk the effects of nausea and vomiting on quality of life? The practice of withholding antiemetic therapy also does not make economic sense. An economic analysis showed that adherence to the guidelines was associated with equivalent or perhaps even lower costs compared with practices before the guidelines were implemented. Additionally, studies have shown that effective combination antiemetic therapy that includes an NK1 antagonist is at least cost neutral and might even be cost saving. Preventing CINV may help avoid much larger expenditures later on, such as use of growth factor support, which is far more expensive than antiemetic therapy,” Dr. Gralla said.
Summary
Antiemetic therapy remains an essential component of cancer care. Effective control of CINV preserves quality of life, permits safe outpatient treatment, reduces or eliminates associated symptoms and side effects, and enhances use of the most effective antineoplastic agents, Dr. Gralla said in conclusion. Newer antiemetics and new clinical trials suggest significant improvement in controlling acute and delayed emesis. The improved control reflects development of agents that target specific receptors associated with emesis and adherence to clinical guidelines that emphasize evidence-based strategies to prevent CINV.