cardiology
American College of Cardiology (ACC) 60th Annual Scientific Sessions
Randomized Comparison Distinguishes PPIs for Relative Effect on Antiplatelet Agent Activity
New Orleans – Some proton pump inhibitors (PPIs) may pose a substantial risk of drug-drug interactions by competing for metabolism through the hepatic cytochrome P450 system. One of the most serious potential interactions is with clopidogrel, an antiplatelet agent that prevents cardiovascular (CV) events. However, a recent randomized comparison of PPIs, not all of which are dependent on hepatic metabolism, demonstrated important relative differences for a clopidogrel interaction. Of PPIs studied on standard dosing, a new agent in this class produced the least antiplatelet impairment, while an older agent produced the most. The findings are relevant to the potentially life-saving effects of clopidogrel in the context of PPIs, which are commonly co-administrated in the many patients with CV disease who need gastroprotection. This comparative study also supplied insight on the heterogeneous pharmacokinetics of clopidogrel, which ranged broadly among subjects even prior to PPI administration.
The antiplatelet agent clopidogrel and proton pump inhibitors (PPIs) are among the most prescribed agents of any kind; they are also often commonly co-administered. The same aging patient with cardiovascular (CV) disease who requires an antiplatelet agent not infrequently also warrants a PPI to reduce the risk of gastric injury from antiplatelet agents and non-steroidal anti-inflammatory drugs (NSAIDs). In a randomized trial of newer and older agents, that concern appears justified. The study proved that PPIs are not interchangeable for their influence on clopidogrel’s antiplatelet effect, a finding that may be relevant to other important drug-drug interactions.
There does appear to be an inadequate appreciation for the fact that PPIs are not interchangeable as demonstrated in this study.
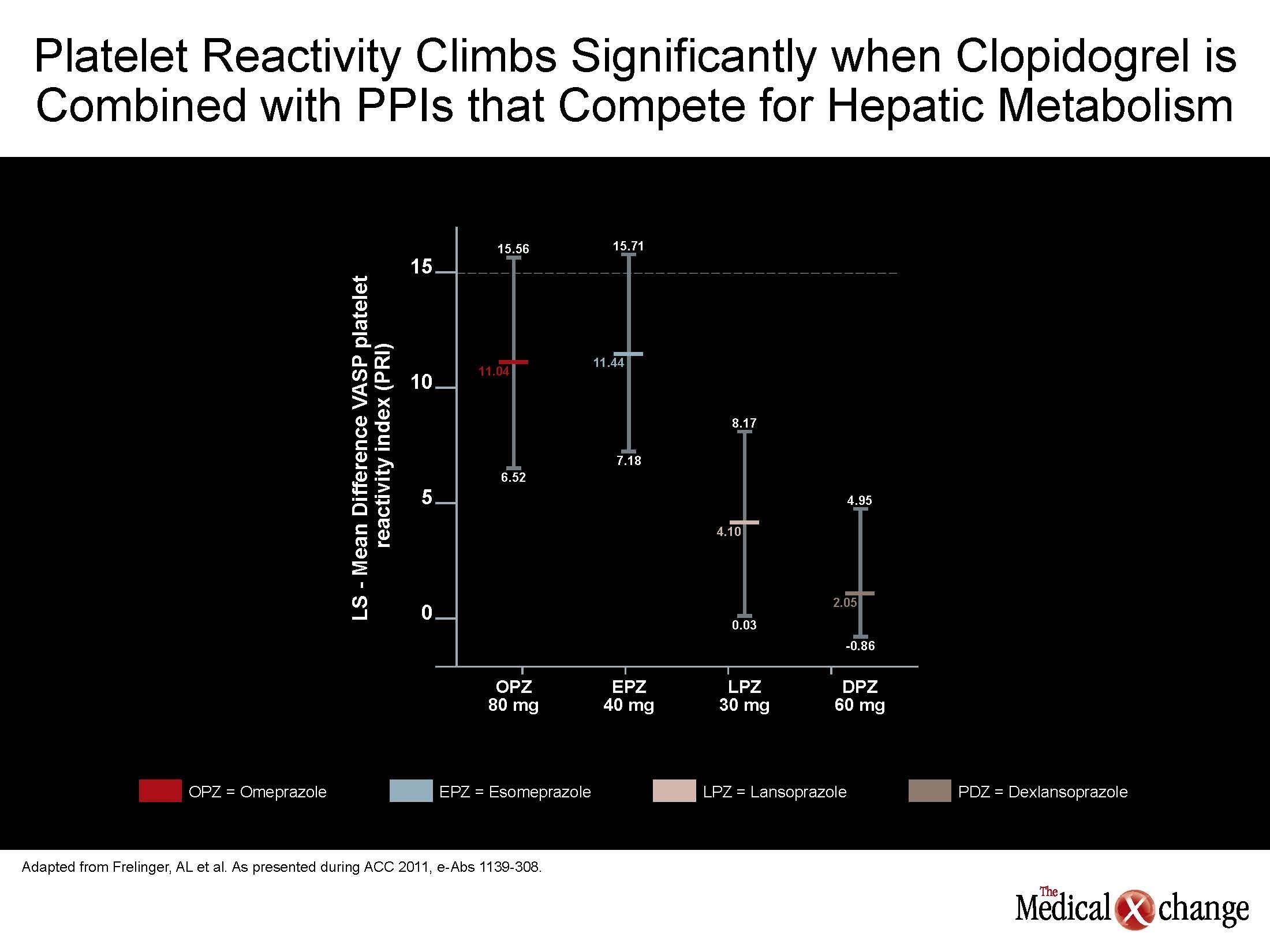
“The bottom line is that PPIs are not interchangeable, and the effects of PPIs on clopidogrel activity are minimized with use of dexlansoprazole or lansoprazole rather than esomeprazole or omeprazole,” reported Dr. Andrew L. Frelinger, III, Associate Director, Center for Platelet Research Studies, Harvard Medical School, Boston, Massachusetts. “There does appear to be an inadequate appreciation for the fact that PPIs are not interchangeable as demonstrated in this study.”
Studying Effects of PPIs on Antiplatelet Agent Activity
This study had two important components: the most important was the comparison of the effect of PPIs on clopidogrel activity. The study included a newer agent, dexlansoprazole, which is unique for a dual delayed release technology that produces two plasma peaks to improve 24-hour acid control, as well as lansoprazole, omeprazole, and esomeprazole. Data were also presented from a baseline evaluation of the inter-individual differences in clopidogrel pharmacokinetics (PK) in the absence of PPIs. An appropriate first step in attempting to understand the PPI comparison, this analysis had its own value in revealing the substantial variability on clopidogrel PK even in the absence of PPIs.
In the study, 160 healthy subjects were evaluated. The subjects were free of nicotine use for at least six weeks, free of alcohol or caffeine use for at least 72 hours, and off all drugs, including over-the-counter agents. In addition, all subjects were homozygous for the CYP2C19 extensive metabolizer genotype, a well-recognized confounder of clopidogrel metabolism.
All PPIs were evaluated in standard doses except omeprazole, which was administered in a dose of 80 mg explicitly to induce an interaction with clopidogrel to provide a positive control. In two study periods, subjects either received 75 mg clopidogrel alone followed by the antiplatelet agent and the assigned PPI for a second study period or these drugs in the opposite sequence. The two 10-day study periods were separated by a washout. Clopidogrel PK were compared with maximal plasma concentration (Cmax) and area-under-the-curve (AUC) drug exposures. Platelet reactivity and aggregation were measured with standard indexes after adenosine diphosphate (ADP) challenge at 5 mcg and 20 mcg concentrations.
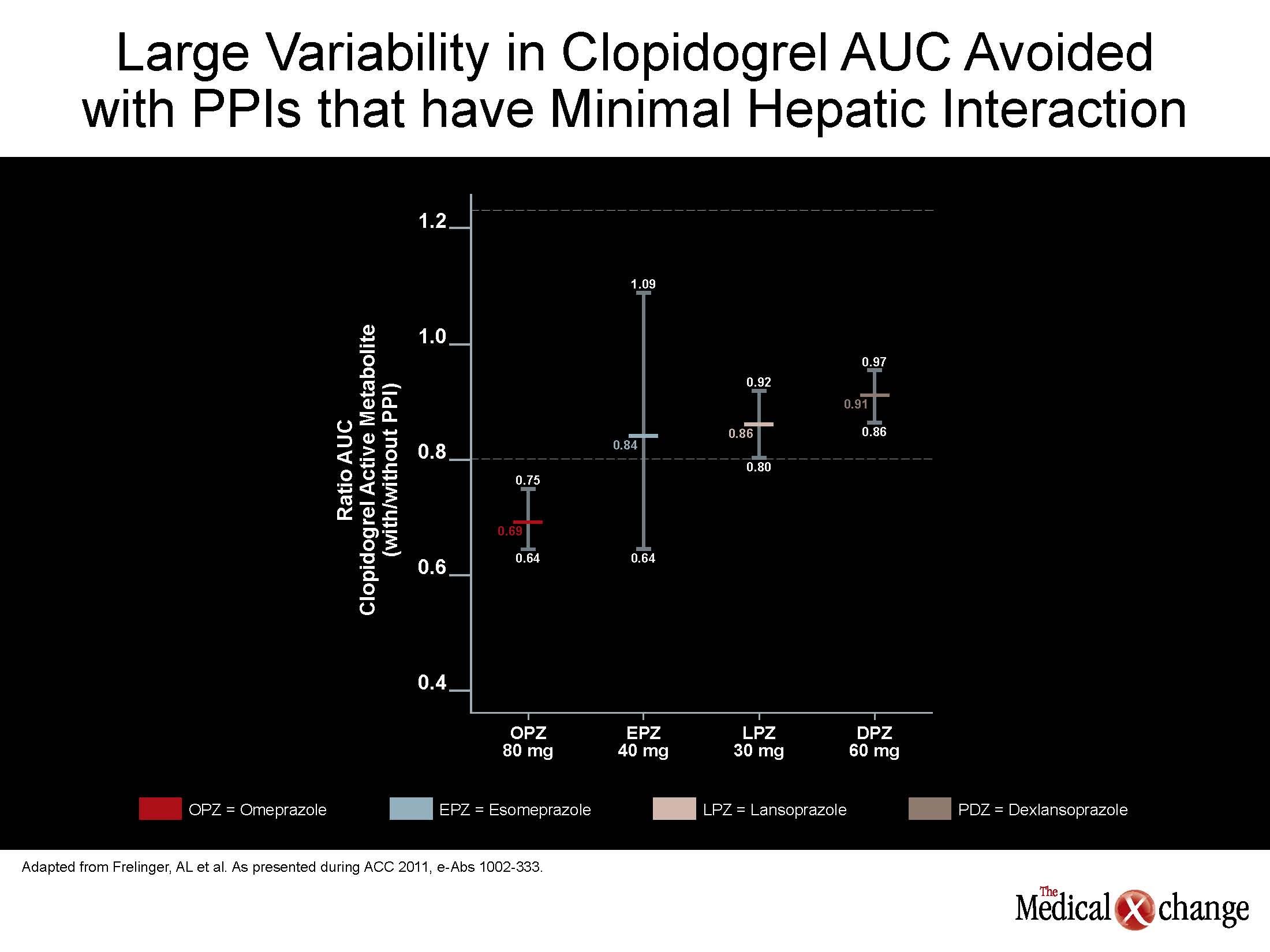
Off PPIs, no measure of PK or platelet function differed across groups. On PPIs, PK changes were greatest on esomeprazole and omeprazole and least on dexlansoprazole and lansoprazole. These differences in effect on PK, such as a relatively small influence on AUC on dexlansoprazole relative to esomeprazole were reflected in difference on the observed antiplatelet effect (see Figure 1) (Fig. 1).
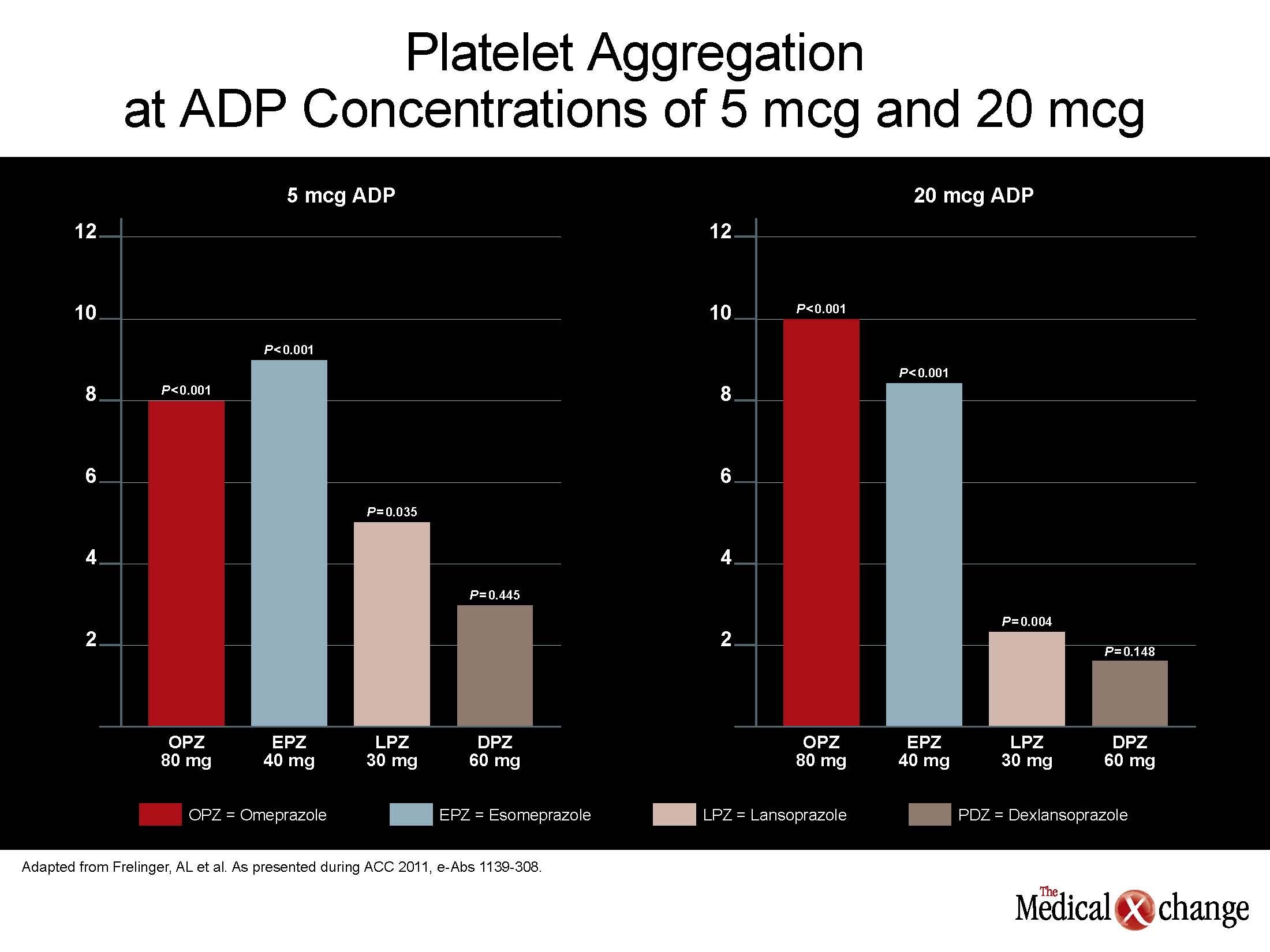
“When clopidogrel was co-administered with esomeprazole 40 mg, the antiplatelet effect was reduced by a similar amount to that of 80 mg omeprazole co-administration,” Dr. Frelinger reported. This was true whether ADP concentration was 5 or 20 mcg. In contrast, neither co-administration of dexlansoprazole nor lansoprazole significantly reduced the antiplatelet effect of clopidogrel at the lower ADP concentration. At the higher ADP concentration, dexlansoprazole was the only PPI which did not significantly lessen the antiplatelet effect of clopidogrel alone (see Figure 2) (Fig. 2).
While these data confirm that PPIs are not interchangeable, the data also indicate that relative acid suppression is not relevant to clopidogrel PK or activity.
While these data confirm that PPIs are not interchangeable, the data also indicate that relative acid suppression is not relevant to clopidogrel PK or activit y. Rather, dexlansoprazole, which is likely to provide the most consistent 24-hour acid control due to its dual-peak plasma concentration, had the least influence on clopidogrel activity. The potential clinical significance of the differences between the PPIs was further reinforced by the baseline analysis which compared platelet reactivity in the subjects prior to PPI exposure. In this part of the study, there was a wide variability in the AUC for the active metabolite of clopidogrel and in degree of platelet inhibition. The platelet inhibition ranged by as much as 35% despite the absence of genetic polymorphisms and other confounders among study participants.
Despite extensive efforts to control for a broad array of variables, such as patient weight or even the accuracy of the laboratory analyses, “we could not account for more than half of the inter-individual variability that we observed,” Dr. Frelinger reported.
This has important implications for pursuing strategies with the least likelihood to further exacerbate variability in clopidogrel response. According to Dr. Frelinger, the potential effects of PPIs on clopidogrel efficacy “could be minimized by a drug such as dexlansoprazole relative to a drug such as esomeprazole.” Although Health Canada issued a warning almost two years ago that stated, “Healthcare professionals should be aware of a potential interaction between PPIs or other drugs that inhibit CYP2C19 and clopidogrel leading to a potential reduction in the clinical activity of clopidogrel,” no randomized comparison of PPIs had been conducted at that time.
Conclusion
The dependence of many PPIs on the hepatic cytochrome P450 system creates a risk for important drug-drug interactions. Of these, an interaction with clopidogrel, which is associated with large reductions in risk of CV events through its antiplatelet effect, is among the most worrisome. A comparison of PPIs that are not dependent on the cytochrome P450 system, such as dexlansoprazole, to those that are, such as esomeprazole, do suggest potentially clinically significant differences. In a randomized comparison that found a substantial impairment of clopidogrel’s antiplatelet effect on esomeprazole, dexlansoprazole did not have a significant effect despite a dual peak PK technology.
Figure 3 (Fig. 3).