Multiple Sclerosis
28th Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS)
Neuroprotective Activity is Rapidly Emerging as a Therapeutic Goal in MS that is Independent of Anti-Inflammatory Activity
Lyon – An important paradigm shift in the understanding of the pathological events of multiple sclerosis (MS) has been strengthened by a large series of diverse and independent presentations at the 2012 ECTRIMS. The premise of this shift is that the neurodegeneration that characterizes progressive MS disability is a process that is independent of focal autoimmune inflammatory activity. While inflammation damages myelin and remains the most likely source of acute symptoms, neurodegeneration produces disseminated damage in the white and gray matter that appears to be a more important driver of sustained disability. The broad spectrum of evidence that supports inflammatory activity and neurodegeneration as independent processes not only includes imaging data and observational studies, but an unexpected separation between protection against relapse and sustained disability that is being observed with novel agents. These data have important implications for long-term disease control.
Damage to the myelin sheaths from autoimmune inflammatory activity has long been understood to be the key pathological event in multiple sclerosis (MS). All currently available licensed therapies are known to suppress or modulate mediators of inflammation. The independent role of neurodegeneration, long suspected by the prognostic value of disseminated injury to the white (WM) and grey matter (GM) independent of markers of inflammation, is becoming better defined. Not least important from the clinical perspective, several experimental agents have demonstrated protection against sustained disability independent of relapse rate. This challenges fundamental concepts of pathology.
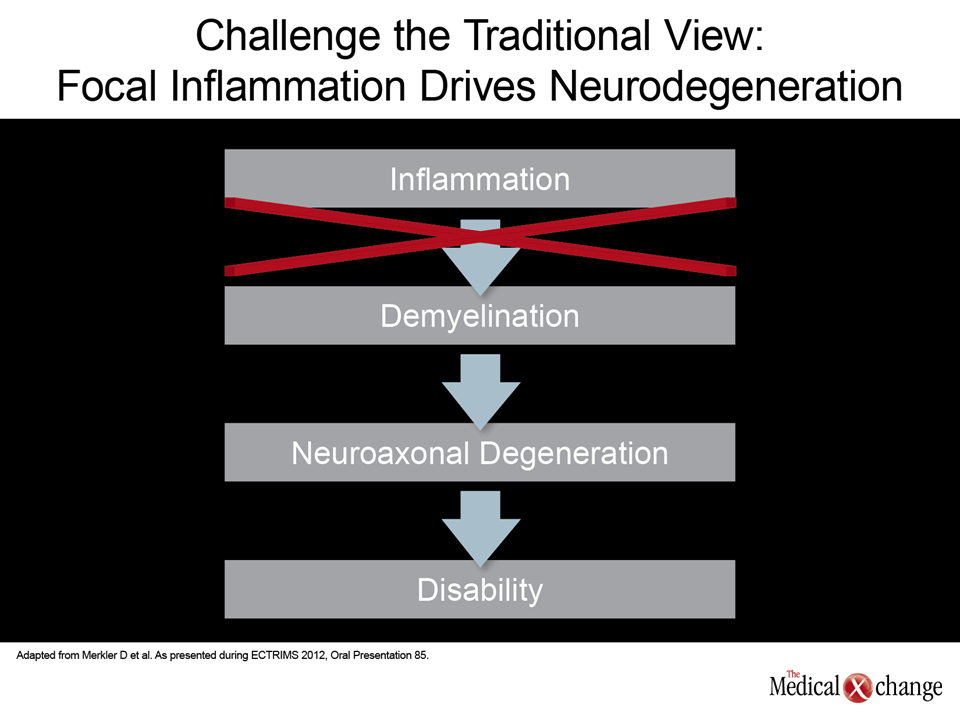
Expanding on Tradition: The Importance of Complex Neurodegenerative Processes
“The prevailing dogma has been that MS can be explained by the upregulation of the inflammatory mediators. Stop the inflammation and stop the downstream events that drive the progression of MS,” explained Dr. Gavin Giovannoni, Chief of Neurology, Blizzard Institute, Barts and the London School of Medicine and Dentistry, UK. The problem is that this premise is not fully consistent with natural history studies or with the activity of newer agents showing benefit in MS. While downstream events triggered by inflammation were thought to include neurodegeneration, Dr. Giovannoni indicated that data support the importance of other, more complex neurodegenerative processes that are initiated at the earliest stages of disease. The same point was made by Dr. Doron Merkler, Department of Pathology and Immunology, University of Geneva, Switzerland. Dr. Merkler reviewed a wide body of evidence that challenges the traditional linear relationship between inflammation and neurodegeneration to propose a more complex process that is likely to involve an interrelationship but independent risk of disability driven by both inflammation and neurodegeneration (Fig. 1). “The rate of clinical relapses only has a marginal effect on the risk of accumulating disability. Rather, the duration of the disease is a much more important relationship to declining EDSS [Expanded Disability Status Scale] scores,” Dr. Merkler observed. He cited several studies that have demonstrated no association between brain atrophy and inflammation as documented with MRI. In addition, the diffuse degeneration that defines brain atrophy in MS has been shown to develop independent of focal demyelinating lesions in relapsing-remitting, primary progressive, and secondary progressive MS.
“The emerging view is that focal inflammation is the tip of the iceberg in understanding MS.”
In the setting of acute relapses, remyelination has been shown to lead to functional recovery, Dr. Merkler acknowledged, but he reported late-stage disability progresses in animal models of MS despite this remyelination, suggesting that therapies effective for preventing disability will need to halt the molecular events that drive neurodegeneration independent of the effect on inflammation. While the role of diffuse inflammation as a pathogenic process may be as important as neurodegeneration in the WM and GM injury that drives sustained disability, Dr. Merkler described a chicken-and-egg phenomenon in which neuronal loss may trigger diffuse inflammation rather than the other way around. In either case, neurodegeneration cannot be ignored. “The emerging view is that focal inflammation is the tip of the iceberg in understanding MS,” Dr. Merkler observed. The trajectory of MS from initial destruction of myelin loss to sustained disability may be difficult to understand when neurodegeneration “is not part of the clinical picture.”
Preventing Diffuse Brain Injury a Key Clinical Goal
The premise that preventing diffuse brain injury may be a more important clinical goal than preventing focal lesions, which has been the focus of clinical trials with conventional agents, was supported by several studies presented at ECTRIMS. Not least of these was one which was designed to identify which MRI markers early in disease were most effective in predicting disability at 10 years. The MRI parameters evaluated in 36 patients with relapsing-remitting MS included lesion load, brain parenchymal fraction, ventricular fraction, and mean magnetization transfer ratio (MTR) of normal appearing brain-tissue. Referring to MTR of the normal appearing brain-tissue, “the MRI parameters that reflected the severity of early diffuse brain damage at the time of diagnosis best predicted the accumulation of disability 10 years later,” reported Dr. Bruno Brochet, Department of Neurology and Neuroimaging, Centre Hospitalier-Universitaire, Bordeaux, France. In contrast, the severity of focal damage, as assessed with ventricular fraction, was a non-significant predictor of disability (Figures 2 (Fig. 2) and 3 (Fig. 3)). The evidence that diffuse disease in the GM and WM correlates with disability and is present at early stages of MS suggests that therapies targeted only at focal inflammation, although they reduce the risk of relapse, may be relatively ineffective in preventing sustained disability. In a presentation by Dr. Wolfgang Brück, Chief, Department of Neuropathology, University Medical Center, Göttingen, Germany, that evaluated the relationship of focal to diffuse damage in MS, he cited a variety of evidence that suggests that the diffuse pathology is driven by different factors than focal damage and therefore should be targeted independently. “The current approved immunomodulatory or immunosuppressive treatments for MS are mainly targeting different cells of the peripheral immune system. However, the residential inflammation in the brain, especially the one occurring in the diffusely abnormal WM, is mainly driven by astrocyte and microglia activation,” Dr. Brück reported. He characterized the focal and diffuse pathologic processes as “largely independent.” The diffuse damage mediated by astrocyte and microglia activation might be attenuated by therapies that effectively downregulate this response or by therapies that provide protection against the neurodegeneration provoked by this upregulation, but current immunomodulating therapies are not considered to act by either pathway. This is important, because Dr. Brück suggested that diffuse disease may be the most important target for strategies to reduce the risk of sustained disability. He cited ongoing work, including his own, of new therapeutic targets with the potential of downregulating astrocyte activation, such as a protein complex that controls transcription of inflammatory genes. Experimental studies conducted with MS drugs in development have demonstrated that activation of astrocytes and microglia can be inhibited by interfering with the NFκB pathway, according to Dr. Brück, an active investigator in this area. Specifically, studies with laquinimod, an oral MS therapy in late stages of clinical testing, has been shown to penetrate the central nervous system (CNS), interfere with the NFκB pathway and reduce axonal loss associated with activated astrocytes. Based on these studies, Dr. Brück reported that this agent appears to act on both inflammatory and neuroprotective pathways, taking the mechanism of action beyond focal inflammation. The same basic conclusion, based on other data, was reiterated by Dr. Massimo Filippi, Neuroimaging Research Unit, Ospedale San Raffaele, Milan, Italy. Dr. Filippi reported that the discrepancy between MRI and clinical findings was the impetus to begin an ongoing prospective study 13 years ago in which progression of disability has been evaluated in the context of baseline MRI findings. Of the 73 patients, data are available on 67. Of potentially important findings, GM damage has emerged as a significant predictor of sustained disability. Although the baseline GM damage, expressed as GM fraction (GMF) when measured with MTR, only trended as a predictor of disability at 13 years of follow-up in a multivariate analysis, the rate of GM damage within the initial 12 months after the initial MRI study, along with duration of MS at the time of the initial MRI study, did emerge as a disability predictor (Fig. 4). “It has been demonstrated previously that GM damage correlates with disability, but these data indicate that the speed of damage is important,” Dr. Filippi reported. The implication of this finding is that therapies effective in preventing disability must act on GM pathology independent of their ability to control the focal MRI lesions that are the target of current therapies. The assertion that prevention of GM damage may be an important mechanism for preventing disability is now supported by clinical data being generated by several experimental MS therapies. In his review of treatment highlights at ECTRIMS, Dr. Giovannoni discussed the implications of what he described as the “disconnect” between control of relapse and control of disability with several such agents. Unlike the experience with conventional disease-modifying agents which typically produce a greater relative reduction in the annualized relapse rate (ARR) than progression of disability when compared to placebo, some of the newer agents are demonstrating a greater reduction in progression of disability than ARR. This is potentially an important advantage in regard to long-term outcome. The data presented at the 2012 ECTRIMS with laquinimod was specifically cited by Dr. Giovannoni as an example of this disconnect. An oral agent that penetrates the central nervous system (CNS), laquinimod was shown in preclinical trials to reduce demyelinating inflammation. In addition, it was shown to modify immune cell activity in the periphery, which is potentially important in preventing the diffuse brain loss that may contribute to progressive disability. So far, clinical studies support a large relative protection against disability progression. In data summarized from the ALLEGRO and BRAVO at the 2012 ECTRIMS, laquinimod was associated with a 46% reduction (P<0.0001) in sustained disability progression at 6 months (Fig. 5). Although laquinimod also provided a highly statistically significant 21% reduction (P=0.0005) in ARR, suggesting substantial activity in preventing demyelinating lesions as measured radiographically, the reduction in progression of sustained disability at 6 months is greater than that observed with other disease modifying therapies in MS. A similar disconnect appears to be emerging in clinical data generated by studies with alemtuzumab, a monoclonal antibody against the CD52 protein on lymphocytes, and daclizumab, a monoclonal antibody directed against the alpha subunit of the IL-2 receptor on T cells, according to Dr. Giovannoni. In one study presented at ECTRIMS, alemtuzumab was associated with a 39% reduction in the progression of sustained disability relative to IFN B-1a. In data presented by Dr. Giovannoni, daclizumab was associated with a 46% reduction in progression of sustained disability relative to placebo. According to Dr. Giovannoni, the reductions in risk of progression of disability are greater with all three agents than those commonly reported in previous studies with disease modifying therapies. “For the first time we are seeing a disconnect in which drugs are showing a different degree of protection against disability that would be predicted by their protection against relapse,” Dr. Giovannoni noted. Citing previously-reported data that control of relapse in the initial phases of MS management has not been correlated with prevention of late stages of disability, he indicated that the new trial data support a paradigm shift in which focal inflammation and disability are recognized as potentially unrelated processes. The impact of this shift on MS treatment would be substantial.
Conclusion
MS has long been characterized as an autoimmune inflammatory disease, but the determinants of sustained disability may involve far more complex pathologic processes. The growing evidence that focal inflammation and diffuse brain injury may progress on separate pathways has the potential to produce fundamental changes in the understanding of this disease. Ultimately, protection against sustained disability has the potential to be a far more important predictor of long-term outcome than relapse activity measured radiographically. The studies with newer agents that are relatively active in reducing diffuse brain activity and lowering rates of sustained disability will permit this prediction to be tested.