Respirology
American Thoracic Society (ATS) 2016 International Conference
In COPD, Renewed Emphasis on Symptom Control Provides Framework for Tailoring Therapy
San Francisco – A debate at the 2016 ATS International Conference that centered on the value of initiating combination therapy in chronic obstructive pulmonary disease (COPD) provoked concern that key therapeutic objectives are being overlooked. As expressed in many guidelines, including those issued by the Canadian Thoracic Society, control of dyspnea and other major COPD symptoms is a critical goal. According to one participant in the debate, it is important not to confuse the established goals in COPD with theoretical gains.
Debate on Individualized Therapy
Pointing out this disconnect, Dr. Bartolome R. Celli, Brigham and Women’s Hospital, Harvard Medical School, Boston, said, “We know COPD to be a disease characterized by irreversible bronchial obstruction, and then we give bronchodilators to see how much they reverse.” This was a direct challenge to his opponent’s proposal of upfront use of dual bronchodilation with a long-acting beta agonist (LABA) and a long-acting muscarinic antagonist (LAMA) as first-line therapy in patients at risk for exacerbations. Dr. Celli cautioned that this approach loses sight of individualized therapy for symptom control. Although dual bronchodilator therapy in patients at risk for exacerbations was proposed on the basis of several sets of data, Dr. Celli’s opponent, Dr. Jadwiga A. Wedzicha, Imperial College, London, UK, focused on the recently-published FLAME study for which she was principal investigator. In FLAME, a once-daily LABA/LAMA treatment was associated with a lower risk of exacerbations at the end of 52 weeks than a twice-daily LABA combined with an inhaled corticosteroid (ICS). This result provided Dr. Wedzicha with her primary argument for preferred use of a LABA/LAMA relative to a LABA/ICS, but Dr. Celli maintained that you cannot advocate the individualized therapy that most feel is essential for treating COPD “and then give everyone the same thing.” Even FLAME, a multinational trial with more than 3000 patients, should not be interpreted as providing a one-size-fits-all solution, according to Dr. Celli. For example, FLAME excluded patients with >600 eosinophils/μL, eliminating at least some of those likely to derive the greatest benefit from the anti-inflammatory effects of an ICS. This relationship between eosinophilia and benefit from ICS has been demonstrated in several studies, including a post-hoc analysis of the WISDOM trial presented at the 2016 ATS. WISDOM, like FLAME, was also a double blind, 12-month trial that recruited a population with a history of exacerbations. In the new analysis, the exacerbation rate ratio was consistently greater in the groups with higher eosinophil counts but maintenance ICS, relative to ICS withdrawal, provided protection.
“Patients with screening eosinophil blood levels ≥4% or ≥300 cells/μL had better exacerbation outcomes with continued ICS.”
“As eosinophil blood levels rose, the relative protection of continued ICS from exacerbations increased, approaching significance at ≥300 eosinophils/μL and reaching significance at ≥400 eosinophils/μL,” reported Dr. Henrik Watz, German Center for Lung Research, Grosshansdorf, Germany, providing support for the premise that COPD therapy should be individualized (Fig. 1). In practical terms for clinicians, the individualization of therapy is best guided by symptomatic response, which is advocated by guidelines in the context of improving quality of life (QOL). Some guidelines do provide treatment recommendations based on clinical groupings, such as the GOLD classifications A, B, C, D, but Dr. Celli argued that there is variability within any such classifications and that clinicians should continue to modify treatments by symptoms.
Following Symptoms to Treat COPD Patients
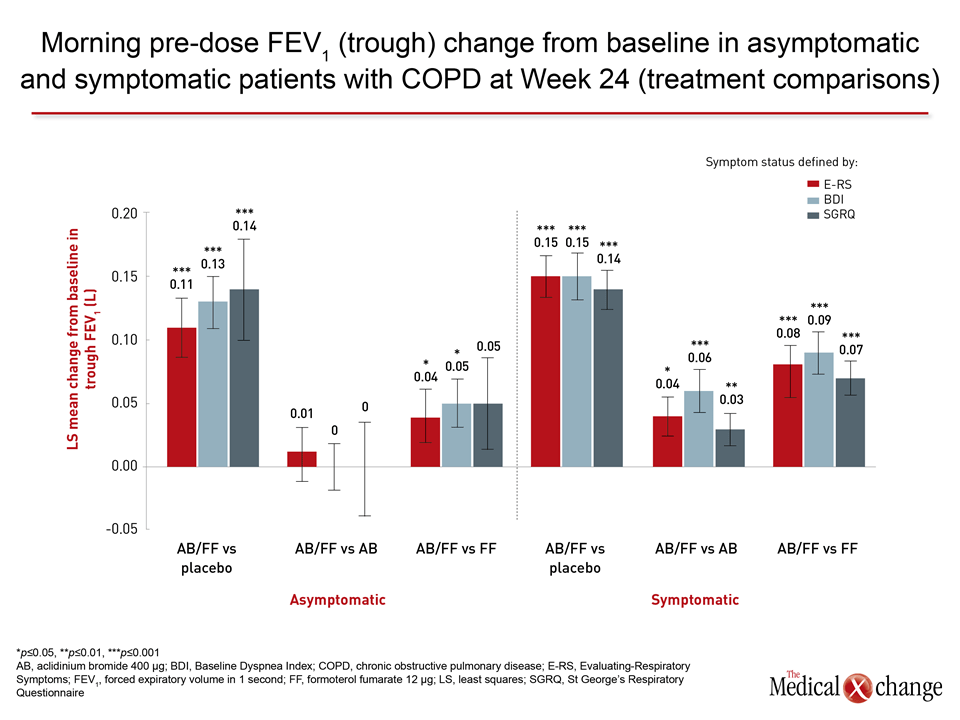
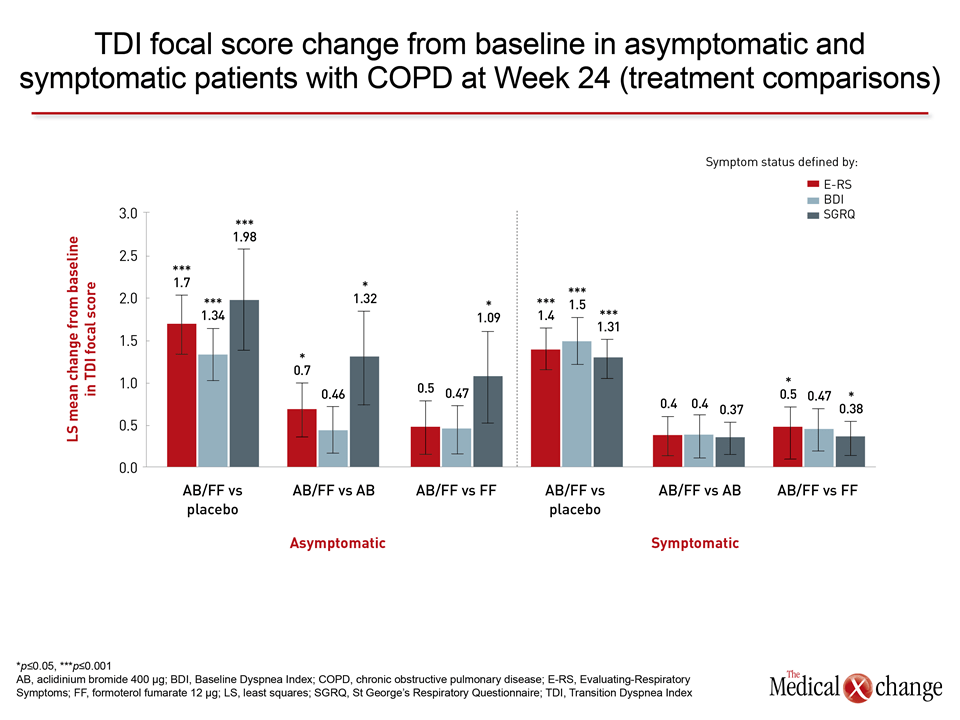
Of symptoms, dyspnea is widely regarded as the most troublesome to patients, but two measures to look at broader outcomes were evaluated from phase 3 trials with the dual bronchodilator combination of the LAMA aclidinium and the LABA formoterol (AB/FF). In pooled data from the ACLIFORM and AUGMENT trials, a composite endpoint of clinically-meaningful endpoints assembled in a tool called Clinically Important Deterioration (CID) was the focus.
“AB/FF improved bronchodilation versus placebo in symptomatic and asymptomatic patients regardless of symptomatic definitions.”
When AB/FF or AB and FF individually were compared to placebo in the pooled data for CID, which includes a dyspnea index, a QOL scoring tool, a sum of exacerbations, and change in lung function, a highly statistically significant advantage was observed for all active therapies relative to placebo in protection from CID (P<0.001) and sustained CID (P<0.001), according to the first author, Dr. Dave Singh, Professor of Respiratory Medicine, University of Manchester, UK (Table 1). He further reported, “Reductions in all the individual components of this composite score were also observed for the LABA/LAMA relative to placebo.” In the second analysis, which, like the first, included 3,394 patients from the pooled studies, bronchodilation endpoints were used to judge benefit while stratifying patients as symptomatic or asymptomatic with multiple definitions. In this study, led by Dr. Marc Miravitlles, University Hospital Vall d’Hebron, Barcelona, Spain, AB/FF improved dyspnea on a validated index relative to placebo even in patients with low baseline dyspnea score, but he emphasized the highly-significant improvement in lung function on multiple measures such as post-dose morning FEV1 (P<0.001). “AB/FF improved bronchodilation versus placebo in symptomatic and asymptomatic patients regardless of symptomatic definitions,” Dr. Miravitlles reported.
Conclusion
The focus on this symptomatic benefit is consistent with the remarks of Dr. Celli, whose main point was that therapy must be tailored for each patient to reduce the burden of COPD. This does not preclude use of dual bronchodilators, but it should not ignore the other proven tools, including ICS, that have been shown effective for symptom control.