Respirology
European Respiratory Society (ERS) International Congress 2016
Results From Multinational Phase 3 Trials: Benefit of Depleting Eosinophils in Severe Asthma Reinforced with New Biologic
London, UK – A new set of data demonstrates for the third time that biologics targeted on the interleukin-5 (IL-5) pathway are effective in patients with otherwise severe uncontrolled asthma. Although the newest agent targets a different point in the IL-5 pathway than the two anti-IL5 monoclonal antibodies currently available in Canada and elsewhere, the registration trials presented at the 2016 ERS Congress associate inhibition of IL-5 activity with unprecedented levels of asthma control. When added to other therapies, the targeted IL-5 pathway inhibitors significantly and substantially reduce the rate of exacerbations and improve lung function, meeting an urgent need for new strategies for asthma poorly controlled on traditional therapies.
Studies Validate Eosinophil Target
The cytokine IL-5 is a major mediator of eosinophil activation, proliferation, and survival. Due to the association of elevated sputum and blood eosinophils with poor asthma control, inhibition of the activity of IL-5 has been a logical strategy for the treatment of asthma that was pursued initially in the experimental setting and now validated with clinical trials. Approximately 50% of patients with asthma have eosinophilic asthma, a phenotype associated with greater asthma severity. In such patients, a positive correlation links rising sputum and blood eosinophil levels with poor asthma control. In poorly controlled severe asthma, the need for new treatment strategies is well recognized. About 10% of asthma patients have severe disease, which is defined by the ERS and others as recurrent exacerbations, airflow limitations, and/or poor symptom control despite high doses of standard therapies. Asthma that worsens when high dose inhaled or systemic corticosteroids are tapered is another criterion for severe disease. In such patients, benefit derived from biologics acting on the IL-5 pathway has been both a clinical advance and a validation of this target. The first phase 3 trial was conducted with mepolizumab, which produced about a 50% reduction in asthma exacerbations relative to placebo over 32 weeks in asthma patients with recurrent exacerbations (Ortega HG et al. N Engl J Med 2014;371:1198-1207). Two phase 3 studies with reslizumab, which enrolled patients with recurrent exacerbations despite medium-to-high doses of inhaled corticosteroids, produced similar reductions in exacerbations relative to placebo over 1 year (Castro M et al. Lancet Respir Med 2015;3:355-366). The most recent phase 3 trials were completed with benralizumab, which, unlike the two agents evaluated previously, targets the IL-5 receptor. The substantial clinical benefits identified in this trial further demonstrate that reversing eosinophilic inflammation through inhibition of the IL-5 pathway is an important option in challenging patients.
“Baseline eosinophil counts and history of frequent exacerbations were predictors of greater treatment response.”
“The trials not only confirm the efficacy and safety of benralizumab, but baseline eosinophil counts and history of frequent exacerbations were predictors of greater treatment response,” reported Dr. Eugene R. Bleecker, Director, Center for Genomics and Personalized Medicine Research, Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina. The two multinational, placebo-controlled phase 3 benralizumab trials, called SIROCCO and CALIMA, were presented at the 2016 ERS Congress and simultaneously published online in The Lancet [September 4; epub ahead of print]. Dr. Bleecker was the principal investigator of SIROCCO.
“From a patient point of view, the rapid observed improvements [with benralizumab] in symptoms and on spirometry suggest validation of targeting the IL-5 receptor.”
The principal investigator of CALIMA, Dr. J. Mark FitzGerald, Head of Respiratory Medicine, University of British Columbia, Vancouver, also emphasized a correlation between clinical benefit and depletion of eosinophils. In SIROCCO and CALIMA, depletion of eosinophils could not be confirmed until the first observation at 4 weeks, but Dr. FitzGerald noted that earlier studies using bronchoscopy showed near complete depletion of eosinophils in the sputum within 48 hours of initiating benralizumab. “From a patient point of view, the rapid observed improvements [with benralizumab] in symptoms and on spirometry suggest validation of targeting the IL-5 receptor,” Dr. FitzGerald said.
Phase 3 SIROCCO and CALIMA Designs
SIROCCO and CALIMA had similar designs and entry criteria. In SIROCCO, 1205 patients were randomized to 30 mg benralizumab every 4 weeks (Q4W) 30 mg benralizumab every 8 weeks (Q8W), or placebo. All treatments were administered subcutaneously as an add-on to standard treatment. In CALIMA, 1306 patients were randomized to the same three arms. For entry, patients were required to have had at least two exacerbations in the previous year while on high-dose inhaled corticosteroids and long-acting beta-2 agonists. Patients were stratified in a 2:1 ratio for eosinophil counts of ≥300 per μL and <300 per μL. Exacerbation rate in patients with eosinophil counts of ≥300 per μL was the primary outcome. Each trial met its primary endpoint. In SIROCCO, the reductions in the exacerbation rate relative to placebo over 48 weeks were 45% and 51% (both P<0.0001) for the Q4W and Q8W schedules, respectively. In CALIMA, the reductions were 36% (P=0.0018) and 28% (P=0.0188) respectively (Fig.1). While acknowledging that the exacerbation rate reductions were less in CALIMA, Dr. FitzGerald noted between study differences in enrolment, particularly the smaller proportion of CALIMA patients with ≥ 3 exacerbations in the year before entry, are likely to explain modest differences in the magnitude of response. “In the subgroup of CALIMA patients who had three or more exacerbations prior to study entry, the reduction in exacerbations relative to placebo for the Q4W and Q8W schedules of benralizumab mirror the reductions observed in the SIROCCO study,” Dr. FitzGerald explained. Consistent with the primary result, benralizumab was associated with at least numerical or significant improvement in key secondary measures, particularly among those with ≥300 eosinophils μL at entry. For lung function, a pre-bronchodilator increase in FEV1 relative to placebo was observed with both schedules and in both trials at the initial 4-week assessment, and this relative improvement was maintained over the course of treatment. Expressed as least-squares mean change from baseline, there was a 0.106 L overall difference (P=0.0215) favouring the Q4W schedule over placebo and a 0.159 L difference (P=0.006) favouring the Q8W schedule in the SIRROCO study. Similar advantages for benralizumab relative to placebo were observed in CALIMA (Fig.2).
Benefit on Symptoms and QOL
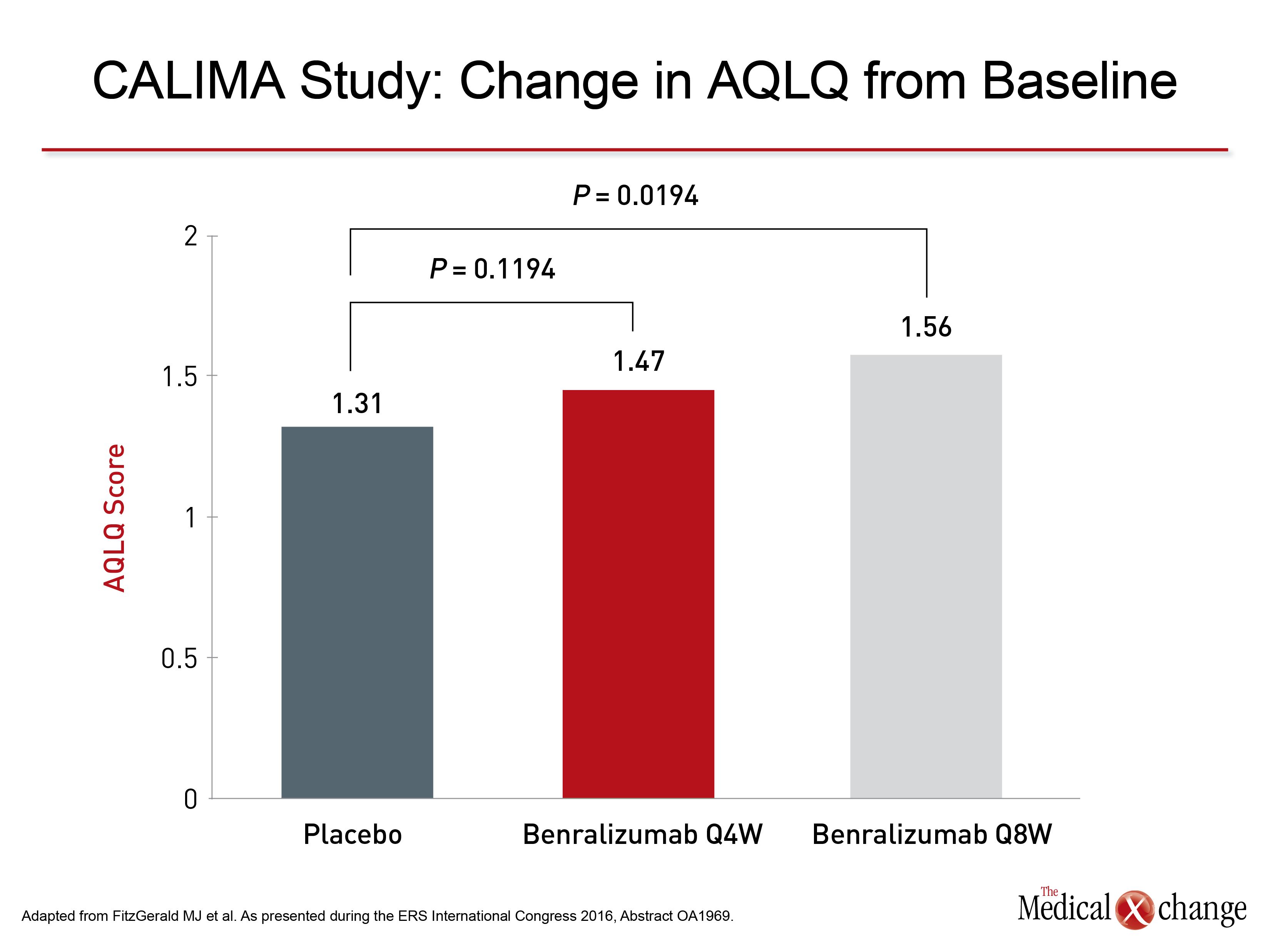
For asthma symptoms, which were evaluated with the six-item Asthma Control Questionnaire (ACQ-6), the advantage was consistently significant among those with higher eosinophil count at entry. In SIROCCO, the advantage was relatively modest in the Q4W group (P=0.04) but highly significant in the Q8W group (P=0.0028). In the CALIMA trial, the symptom score advantage of benralizumab relative to placebo approached significance with the Q4W schedule in the lower eosinophil strata (P=0.0776), was modestly significant in the higher eosinophil group on the Q4W schedule (P=0.0425) and was highly significant on the Q8W schedule (P=0.0082). Similar findings were observed for quality of life, which was evaluated with the Asthma Quality of Life Questionnaire (AQLQ). In the stratum with the higher baseline eosinophil count, the advantage of benralizumab relative to placebo in the SIROCCO study approached significance for the Q4W schedule (P=0.081) and was highly significant for the Q8W schedule (P=0.0036). In CALIMA, the advantage of the Q4W schedule also fell short of significance (P=0.1194) but was significant for Q8W (P=0.0194) (Fig.3). For the primary endpoint of exacerbations, the curves capturing the cumulative number of exacerbations diverged in favour of benralizumab over placebo by 4 weeks, which was the first observation period, in both trials. The time to first asthma exacerbation was also longer for benralizumab relative to placebo and the probability of having an asthma exacerbation was reduced. In the SIROCCO trial, for example, the reduction in probability of an exacerbation was 37% (P=0.0005) for the Q4W schedule and 40% (P=0.0002) for the Q8W schedule.
Benefit and Eosinophils Correlate
Consistent with the mechanism of action, greater benefit was typically observed in those with the highest level of eosinophils at entry. For the primary endpoint of exacerbations in the SIROCCO trial, for example, the percentage reduction in the rate of exacerbations climbed from 30% among those in the lower stratum of baseline eosinophils to 45% among those in the higher stratum. For the Q8W schedule, the reductions in the low and high strata were 17% and 51%, respectively. When other outcomes were stratified by baseline eosinophil count, such as improvement in FEV1, greater relative benefit was commonly observed among those with ≥300 eosinophils μL relative to those with fewer.
Tolerability Profile
In both SIROCCO and CALIMA, benralizumab was well tolerated. In SIROCCO, the rates of any adverse events (76%) and any serious adverse events (14%) were both slightly higher for placebo, than for the Q4W schedule (73% and 12%, respectively) or the Q8W schedule (71% and 13%, respectively). In CALIMA, the rate of serious adverse events was also numerically lower on either benralizumab schedule relative to placebo. No specific side effects, including injection site reactions, were observed at substantially higher rates in the active treatment arms relative to placebo in either study (Table 1).
Targeting the IL-5 Receptor
There are no direct comparisons for any of the monoclonal antibodies targeting the IL-5 pathway. There are, however, theoretical advantages for targeting the IL-5 receptor rather than the cytokine, according to Dr. FitzGerald. He explained that targeting the receptor appears to account for the rapid and near complete eosinophil depletion observed on benralizumab. He suggested that the correlation between blood eosinophil count and clinical outcomes argues for strategies to maximize eosinophil count reductions, particularly in the asthma phenotype that appears to be driven by eosinophilic inflammation. New evidence in support of eosinophil-directed therapy, particularly in patients with severe and persistent asthma, was derived from a cost analysis at the 2016 ERS Congress. In a cohort study using administration healthcare utilization data in 2,392 patients with persistent asthma, there was a significant stepwise increase in incremental asthma-related cost when patients were stratified by eosinophil count ≥150 μL vs. <150 μL, ≥300 μL vs. <300 μL, and ≥400 μL vs.<400 μL. “These findings further emphasize the importance of elevated blood eosinophil count on asthma burden by correlating costs and cell counts,” reported a team of investigators that included Dr. Robert S. Zeiger, Department of Research and Evaluation, Kaiser Permanente Medical Center, San Diego, California.
“The correlation of costs and eosinophil counts “further emphasize the importance of elevated blood eosinophil count on asthma burden.”
Canadian data have also suggested an inverse relationship between asthma control and cost. In a study undertaken in British Columbia, for which Dr. FitzGerald served as senior author, asthma patients with poorly controlled symptoms consumed 94% of asthma-related resource use, which included medications, physician visits and hospitalizations (Sadatsafavi M et al. Can Respir J 2010;17:74-80). These types of data suggest that better options to control disease can not only relieve the high disease burden for patients with severe asthma but control the disproportionate resources allocated to this group.
Conclusion
Benralizumab, as well as the phase 3 studies with mepolizumab and reslizumab, provide convincing evidence that eosinophils are an important target at least in patients with the asthma phenotype driven by an upregulation of eosinophils. These data confirm that targeted therapy at key drivers of the inflammatory component of asthma can provide major clinical benefits.