Cardiology
ACC.17: 66th Annual Scientific Session & Expo
Reduced All-Cause Death Suggests SGLT-2 Inhibitors May Be Part of New Anti-Diabetes Standard
Washington, DC – In contrast to other therapies for type 2 diabetes mellitus (DM2), agents in the SGLT-2 inhibitor class prevent heart failure and reduce all-cause mortality, according to a major study comparing the presence of absence of agent in patients on anti-diabetic drugs. The data are potentially practice changing. Preventing progressive cardiovascular (CV) disease is the most important long-term goal of DM2 therapy. The relative reduction in all-cause death for those initiated on a SGLT-2 inhibitor relative to any other anti-diabetic agent was 51% (P<0.001). This is the second major study to demonstrate these benefits but the first to confirm a class effect.
SGLT-2 Inhibitors and Improved CV Outcomes
Since 2013, inhibitors of the sodium-glucose co-transporter 2 (SGLT-2) protein have been rapidly integrated into the care of DM2. These oral agents inhibit a protein critical to renal glucose reabsorption, are highly effective alone or in combination with other anti-diabetic agents, and are exceptionally well tolerated. The EMPA-REG OUTCOME trial established a SGLT-2 inhibitor as the only anti-diabetic therapy ever to be associated with improved CV outcome in a multicenter randomized trial. The new CVD-REAL study demonstrates this benefit extends to all available SGLT-2 inhibitors.
“In a broad population of patients with DM2 in general practice, SGLT-2 inhibitors provide protection against hospitalization for heart failure and all-cause death.”
“CVD-REAL demonstrated that in a broad population of patients with DM2 in general practice, SGLT-2 inhibitors provide protection against hospitalization for heart failure and all-cause death when compared to other glucose-lowering therapies,” reported Dr. Mikhail Kosiborod, Professor of Cardiology, University of Missouri, Kansas City School of Medicine. “Only a minority of patients had known CV disease, suggesting that benefits extend to those at relatively low baseline risk.”
The CVD-REAL Study
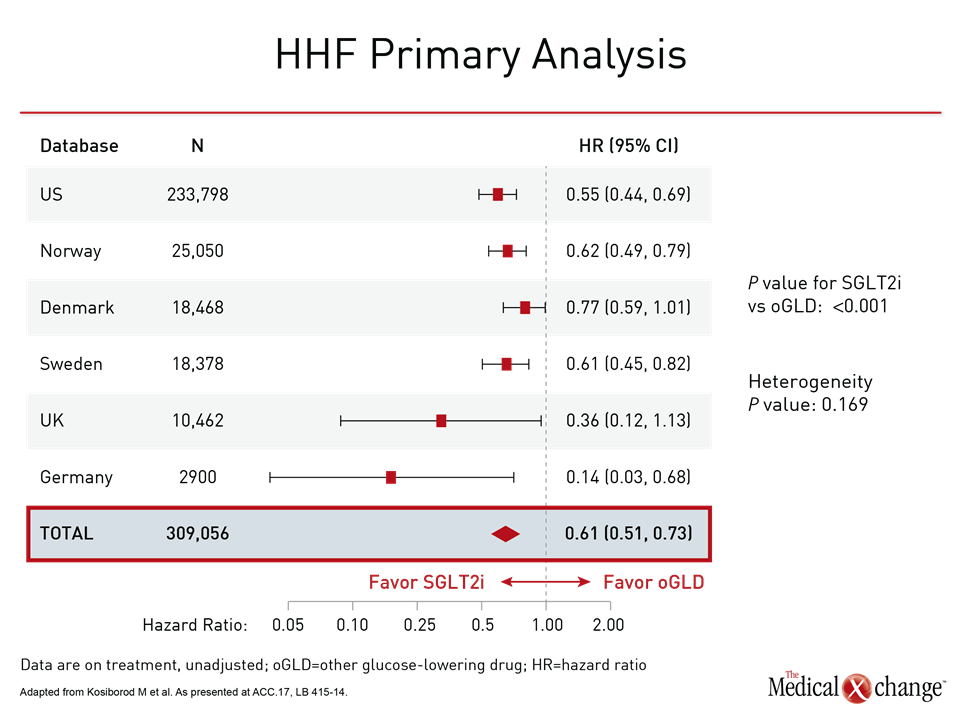
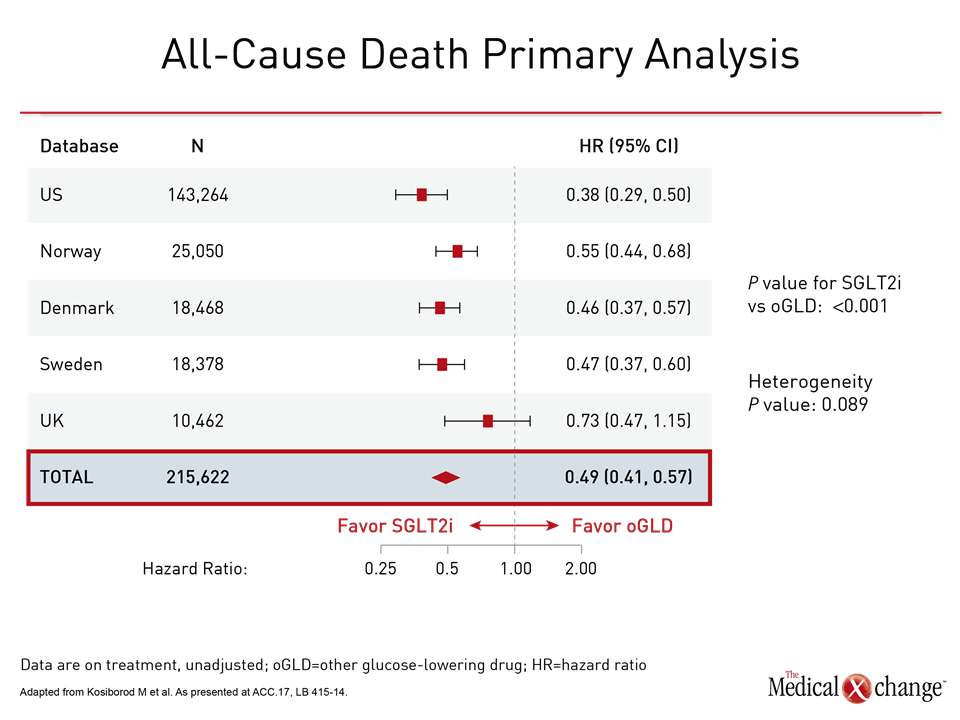
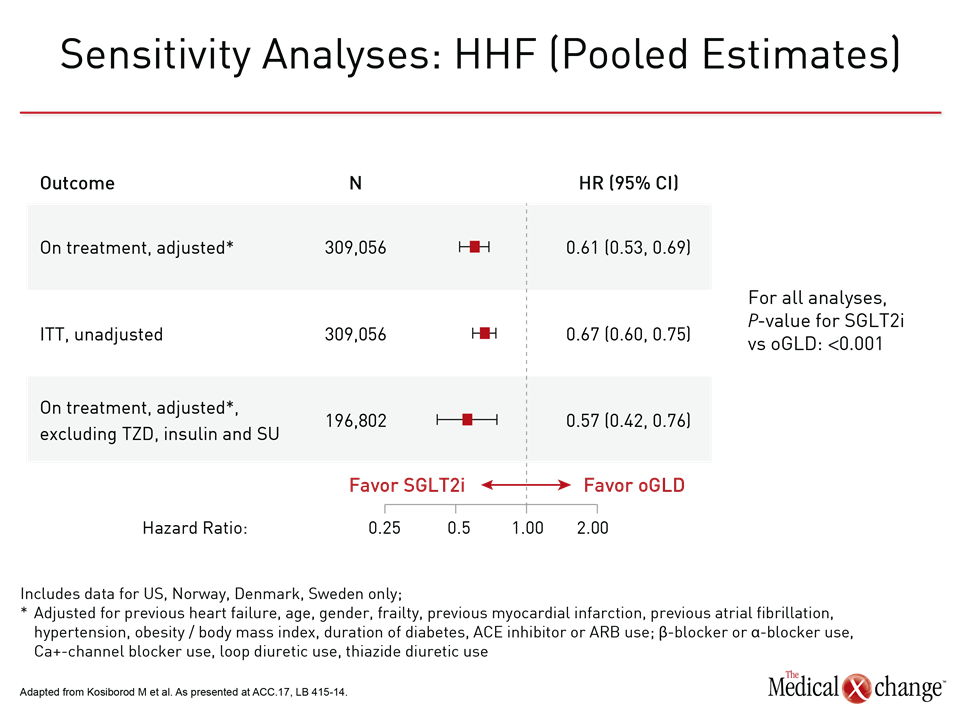
CVD-REAL was conducted with data from real-world patients captured from registries maintained in six countries (US, UK, Germany, Norway, Sweden, and Denmark). New users of glucose-lowering therapies were compared in detailed propensity matching validated through multiple sensitivity analyses. Data were available for 154,523 patients initiating SGLT-2 inhibitors matched to an equal number of patients initiating another antidiabetic therapy. Most were already on background therapy, such as metformin. The primary objective was to compare the impact of these therapies on risk of hospitalization for heart failure. The relative effect on all-cause death was a secondary outcome. Benefits favoring SGLT-2 inhibitors were large and consistent. The hazard ratio (HR) for hospitalization of heart failure of 0.61 (P<0.001) signified a 39% reduction (P<0.001) in relative risk over the course of 175,000 person years of follow-up. The confidence intervals were narrow (95% CI 0.51, 0.73). In a by-country analysis, the results were fully consistent and statistically significant or nearly significant across all registries (Fig. 1). The HR for all-cause death in favor of SGLT-2 inhibitors was even greater (HR 0.49; P<0.001) with a tighter confidence interval (95% CI 0.41, 0.57). These narrow confidence intervals were repeated in the by-country analyses, which were again consistent. They were statistically significant for every country except the UK, where the low number of events (80 deaths vs. ≥250 deaths for each of the other countries) widened the confidence intervals (Fig. 2). Mortality data were not gathered in the German registry, so this population was omitted from this analysis. A combined outcome of hospitalization for heart failure and all-cause death, which was also prespecified, produced results consistent with each outcome separately, generating an HR of 0.54 (P<0.001) in favor of the SGLT-2 inhibitors. All data generated by the three preplanned sensitivity analyses were highly consistent. These were an on-treatment (OT) analysis, an intention-to-treat (ITT) analysis, and an OT analysis comparison with the stepwise exclusion of insulin, sulfonylureas, and thiazolidinediones (TZDs). For the primary endpoint of heart failure, for example, the HRs in favor of SGLT-2 inhibitors for these analyses were 0.61, 0.67, and 0.57 (all P<0.001), respectively (Fig. 3). Due to the propensity matching in this very large patient sample, baseline characteristics were identical or nearly identical for those initiating SGLT-2 inhibitors relative to another glucose-lowering drug. In both groups, the average age was 57 years, 40% were women, and 13% had established CV disease. Of those with established CV disease, only 3.1% had heart failure. Other CV diseases at baseline included prior myocardial infarction (3.1%) stroke (4.1%), and peripheral artery disease (3.4%).
Expanding upon EMPA-REG OUTCOME
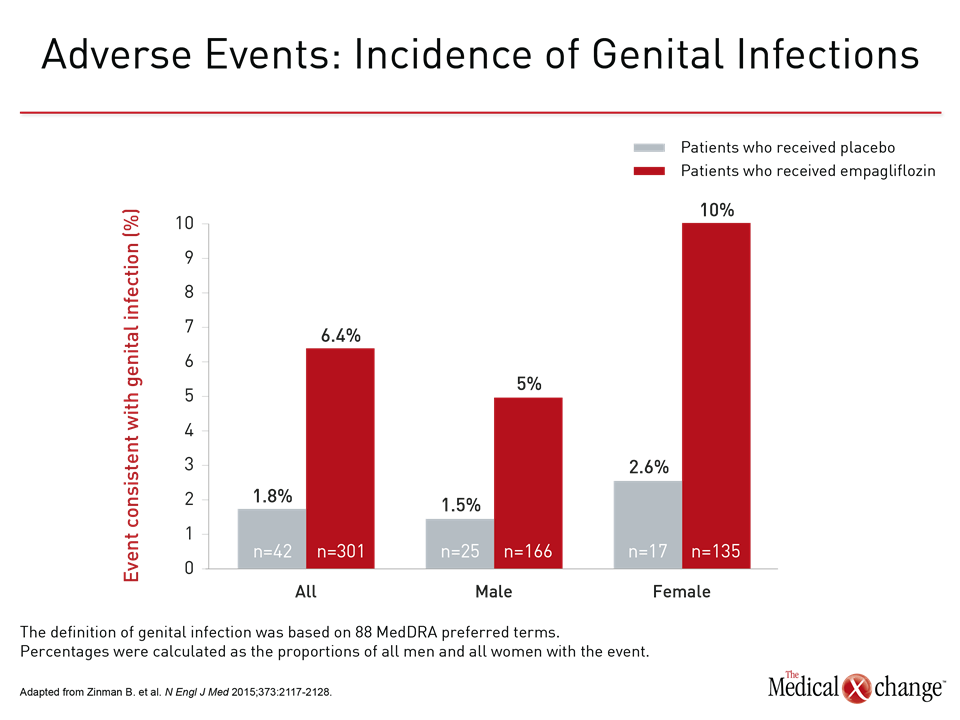
These data greatly extend and expand on the results of the multicenter EMPA-REG OUTCOME trial, which established protection from CV events with the SGLT-2 inhibitor empagliflozin (Zinman B. et al. N Engl J Med 2015;373:2117-2128). In that study of 7,020 patients treated for a median of 3.1 years, the SGLT-2 inhibitor was associated with a 14% reduction (HR 0.86; P=0.04) relative to placebo in the primary composite outcome of death from CV causes, myocardial infarction (MI), or nonfatal stroke. However, in EMPA-REG OUTCOME, patients were required to have established CV disease at entry. “Unlike EMPA-REG OUTCOME, the overwhelming majority of patients—87%—did not have known CV disease at entry,” Dr. Kosiborod emphasized. “This suggests a protective effect rather than a treatment effect.” CVD-REAL did not include a safety analysis, but phase 3 studies have confirmed that these agents have a placebo-like adverse-event profile with the exception of an increased rate of genital infections. These infections, the risk of which can be reduced with more rigorous hygiene, are attributed to the increased excretion of glucose in the urine. In EMPA-REG OUTCOME, as in other SGLT-2 trials, the risk of genital infections was higher in women (Fig. 4).
EMPA-REG OUTCOME vs. CVD-REAL: Outcomes
Despite the differences in the CV risk status of the enrolled populations, the outcomes of EMPA-REG OUTCOME and CVD-REAL showed similar patterns. In EMPA-REG OUTCOME, as in CVD-REAL, the reductions were highly significant for empagliflozin relative to standard therapy for in hospitalization for heart failure (HR 0.65; P=0.002) and all-cause death (HR 0.68; P<0.001). In addition, empagliflozin was associated with a significant protection from death from CV causes (HR 0.62; P<0.001). No significant protection was seen for empagliflozin against other CV events.
Clinical Implications of CVD-REAL
In an analysis of the clinical implications of CVD-REAL, Dr. Kosiborod emphasized three points relevant to SGLT-2 inhibitors. The first is protection from SGLT-2 inhibitors appears to be a class effect. The second is the greatest benefit of SGLT-2 inhibitors may be protection against development of heart failure rather than the reversal of existing pathology. The third is that CV protection from SGLT-2 inhibitors appears to accrue from early use. “The low rate of existing CV disease at baseline in CVD-REAL suggests that benefits may extend to those at the lower end of the risk spectrum,” Dr. Kosiborod said. He implied that these data, like EMPA-REG OUTCOME, provide a powerful rationale for employing SGLT-2 inhibitors in standard management of DM2.
“The low rate of existing CV disease at baseline in CVD-REAL suggests that benefits may extend to those at the lower end of the risk spectrum.”
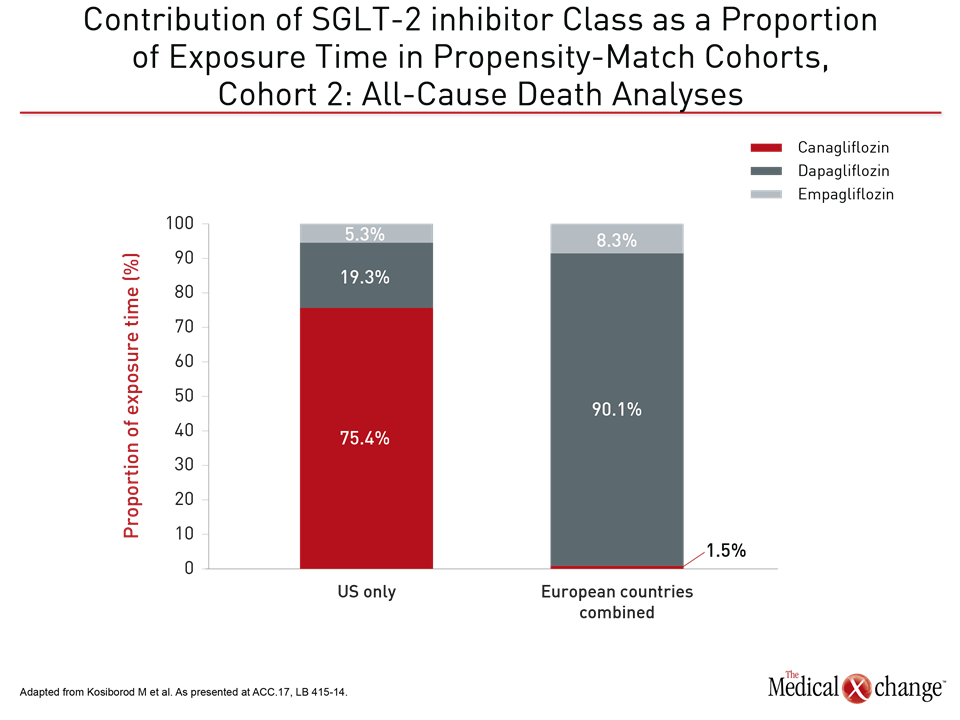
Complying with an FDA mandate that requires CV safety data for antidiabetic drugs, there are on-going trials similar to EMPA-REG OUTCOME for each of the available SGLT-2 inhibitors. In the case of dapagliflozin, for example, randomization in a trial called DECLARE-TIMI58 began in 2013. The outcome of this trial, which has randomized more than 17,000 patients with DM2, is expected in 2019. The CANVAS trial with canagliflozin, which randomized approximately 4,500 DM2 patients, is nearing completion with results expected this year. In CVD-REAL, the proportional exposure times for SGLT-2 inhibitors in the analysis were approximately 11% for empagliflozin and approximately 45% for the remaining two SGLT-2 inhibitors, but dapagliflozin, representing 91.9% of exposure time, was by far the dominant SGLT-2 inhibitor employed in the five European countries while canagliflozin, representing 75.9% of exposure time, was the dominant SGLT-2 inhibitor in the US (Fig. 5). These large differences in exposure relative to the very homogeneous protection against heart failure hospitalizations and all-cause mortality provide the basis for declaring a class effect. In addition to the preplanned sensitivity analyses, several additional post-hoc strategies were employed to rigorously test these findings. This included a technique that involved employing negative controls to look for unexpected and inconsistent data that might suggest imbalances in the data, according to Dr. Kosiborod. He reported that no anomalies using these types of analyses were identified. They will be included in the planned publication of CVD-REAL. A panel of experts assembled at the ACC meeting to critique these results was receptive to the findings. Speaking rhetorically, Dr. Adrian F. Hernandez, Professor of Cardiology, Duke Clinical Research Institute, Durham, North Carolina, said, “Given these results—and we know there are several trials on-going with SGLT-2 inhibitors in regard to CV risk—should we just tell people not to enroll?” Although the remark was facetious, Dr. Kosiborod responded seriously, “We need more trials to drive this message home, because this class of drug really has a remarkable potential to transform patient outcomes.”
“We need more trials to drive this message home, because this class of drug really has a remarkable potential to transform patient outcomes.”
The focus placed in CVD-REAL on heart failure over other CV events, such as MI and stroke, was intentional. Dr. Kosiborod pointed out that many of the favorable effects associated with SGLT-2 inhibitors beyond glucose control, such as weight loss and reductions in blood pressure, arterial stiffness, and vascular resistance, are relevant to protection against heart failure, while heart failure “is arguably the most common and morbid CV complication in DM2.” Protection against heart failure would be expected to lead ultimately to protection against downstream complications, such as arrhythmias. “We have a little bit of data already that patients with diabetes who develop heart failure may be different that the heart failure patients who develop diabetes,” said Dr. Mariell Jessup, Professor of CV Medicine, University of Pennsylvania, Philadelphia. Another expert serving on the ACC panel that critiqued the CVD-REAL results, Dr. Jessup added, “I would second the idea that we need to forge ahead to really understand about what these drugs are doing to improve outcomes.”
“I would second the idea that we need to forge ahead to really understand about what these drugs are doing to improve outcomes.”
Early Use of SGLT-2 Inhibitors
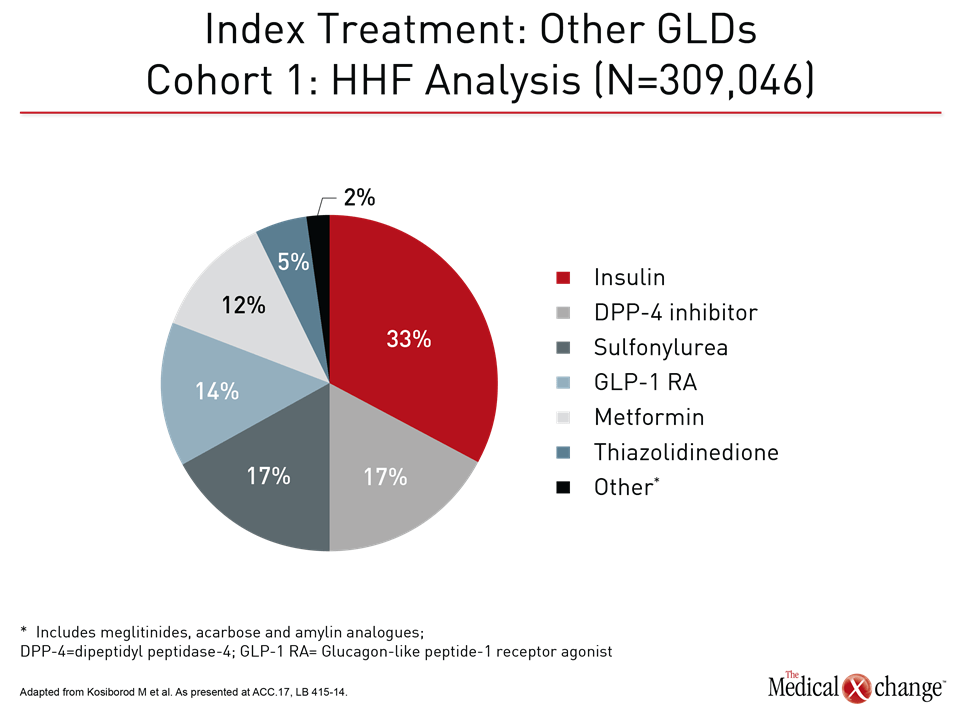
There is now a broad array of agents available for glucose control in DM2. In CVD-REAL, a breakdown of index therapies emphasizes this point (Fig. 6). While essentially all patients in both the SGLT-2 inhibitor group and the matched cohort received combination glucose-lowering therapies, including the nearly 80% in both groups that received metformin, six classes of antidiabetic therapy were represented among index drugs. CVD-REAL, by recreating the benefits observed in EMPA-REG OUTCOME, provides evidence that SGLT-2 inhibitors should be considered early in combination strategies for glucose control.
Conclusion
The EMPA-REG OUTCOME trial was the first randomized controlled trial to associate a glucose-lowering therapy with a reduction in CV events. The CVD-REAL trial suggests that this benefit is a class effect. Together, these results have major implications for selection of treatments early in the management of DM2 for which CV protection is the most important long-term treatment goal.