Cardiology
ACC.17: 66th Annual Scientific Session & Expo
PCSK9 Inhibitors Outcomes Data: Definitive CV Protection
Washington, DC – Several messages emerged with new PCSK9 inhibitor outcome data presented at the 2017 annual meeting of the ACC. The most important is that there is now definitive evidence that cardiovascular (CV) risk reductions correlate with the large reductions in low-density lipoprotein cholesterol (LDL-C) routinely achieved with these agents. The second is that lowering LDL-C levels beyond those possible with statins alone—and beyond current guidelines—provides additional CV protection in high-risk patients. The third is that these agents are remarkably safe even when administered over a period of years and when very low LDL-C levels are reached.
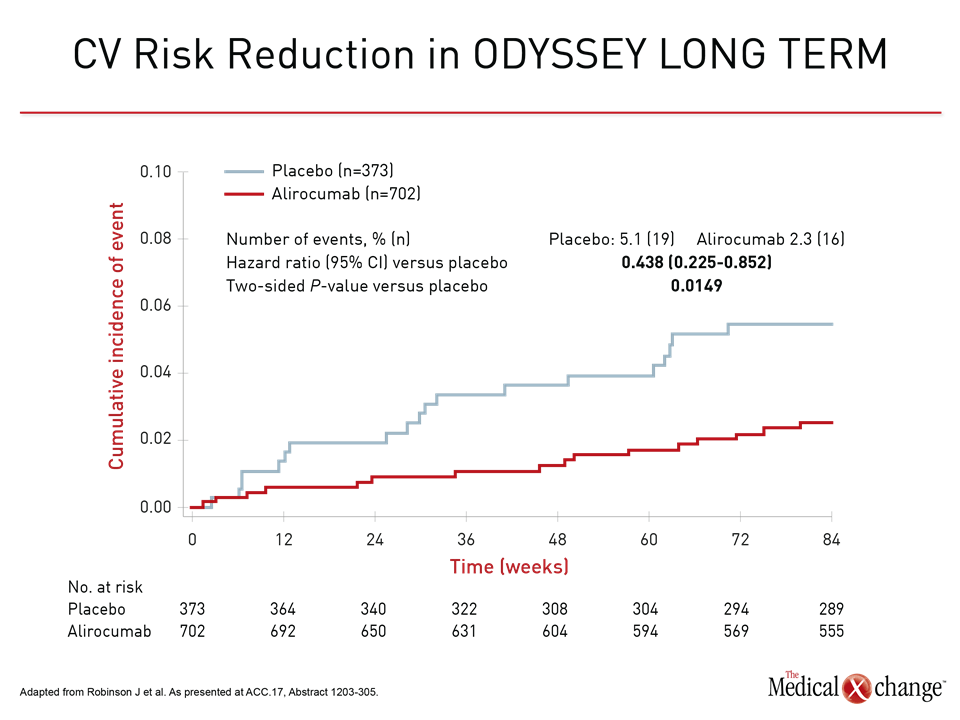
Two major sets of outcome data with the two currently approved PCSK9 inhibitors, alirocumab and evolocumab, provided the support for these conclusions. In new data from ODYSSEY LONG TERM, alirocumab was associated with a 56% reduction relative to placebo in major adverse CV events (MACE) defined as coronary heart disease (CHD), nonfatal myocardial infarction (MI) ischemic stroke, or unstable angina requiring hospitalization (Fig. 1). The 1075 patients in this analysis had atherosclerotic CV disease (ASCVD) plus one additional risk factor.
PCSK9 Inhibitors Yield Event Reductions
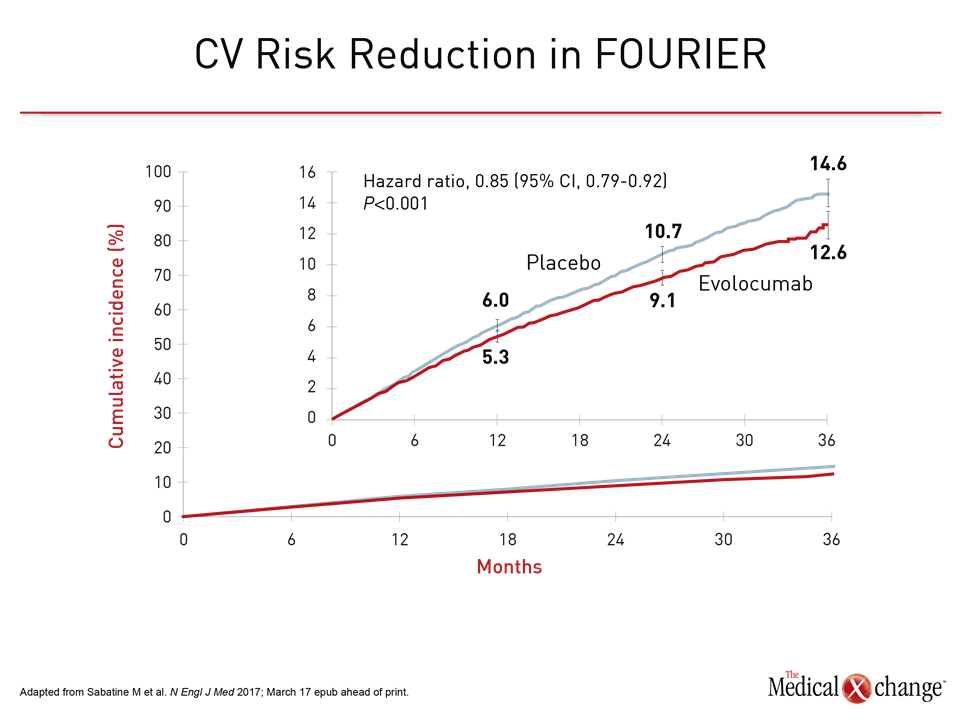
In the other set of data, evolocumab was associated with a 15% reduction (95% CI 8% – 21%) relative to placebo in the primary MACE endpoint of CV death, MI, stroke, hospitalization for unstable angina, or coronary revascularization in the phase 3 FOURIER trial (Fig. 2). The trial randomized 27,564 ASCVD patients and had a median duration of follow up of 2.2 years
The alirocumab and evolocumab data are considered mutually reinforcing and not necessarily different. Some or all of the greater relative risk reduction observed in ODYSSEY LONG TERM may be explained by the higher mean entry LDL-C level in ODYSSEY LONG TERM versus the FOURIER trial (3.06 vs. 2.39 mmol/L) or other disparities in the types of patients each trial enrolled. Another ongoing trial with alirocumab called ODYSSEY OUTCOMES will have lower average LDL-C at enrollment (2.3 mmol/L, according to baseline data presented at this year’s ACC), but there will also be significant differences between this study and FOURIER. Not least, patient enrollment after a CV event will be earlier and follow-up will be approximately twice as long. This is expected to yield more information about the relative value of PCSK9 inhibitors for both early and late CV protection.
“For ODYSSEY OUTCOMES, patients were enrolled 4 to 52 weeks after an ACS, producing a more immediate at-risk population than that of FOURIER.”
“In ODYSSEY OUTCOMES, patients were enrolled four to 52 weeks after an acute coronary syndrome, whereas FOURIER tended to enroll patients later after qualifying events,” explained Dr. Shaun Goodman, Division of Cardiology, St. Michael’s Hospital, Toronto, Canada. He suggested that ODYSSEY OUTCOMES as a result might provide a more detailed analysis of late protection against an array of CV events not just those directly linked to atherosclerosis.
Benefits Consistent Across All Subgroups
Data from the FOURIER trial were made available online on the day that they were presented at the ACC (Sabatine M. et al. N Engl J Med 2017; March 17 epub ahead of print). In addition to the 15% reduction in the hazard ratio (HR) for the primary endpoint (HR 0.85; P<0.001) there was a 20% reduction in the key composite secondary endpoint of CV death, MI, or stroke (HR 0.80; P<0.001). The benefits were consistent across subgroups defined by age and sex. The relative CV protection was also similar in patients with the lowest quartile of LDL-C at entry, which was 1.9 mmol/L, as those with higher levels. It is notable that 1.9 mmol/L is lower than the level once identified in guidelines as the treatment target. The median LDL-C reduction relative to placebo in the evolocumab arm at the end of 48 weeks was 59%.
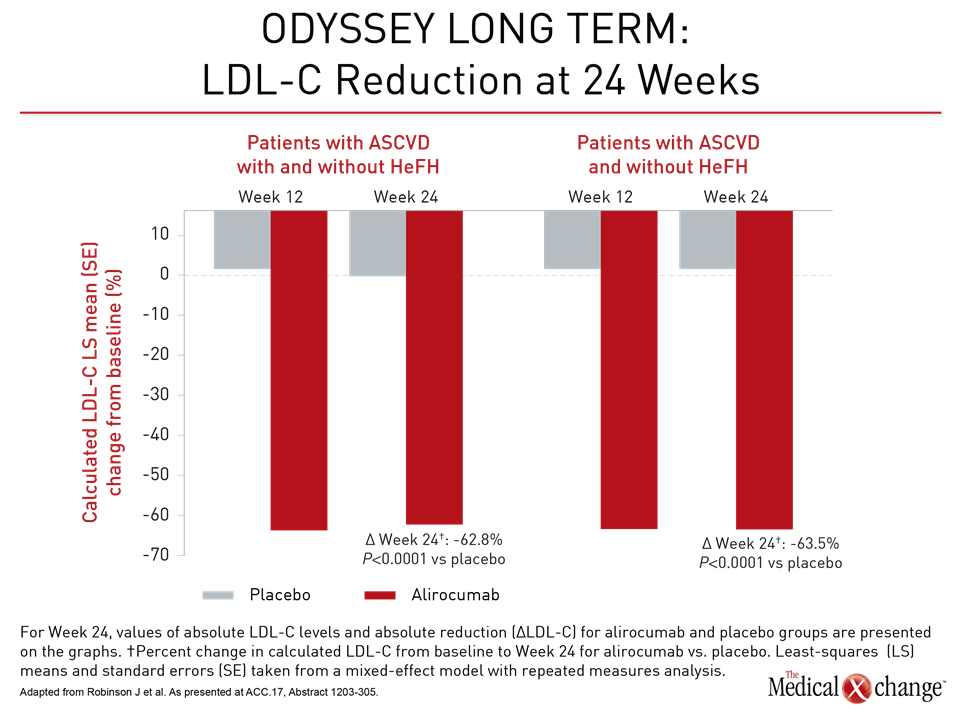
In ODYSSEY LONG TERM, the LDL-C reduction at 24 weeks for alirocumab versus placebo was 62.8%. For the 8% of patients in this analysis with heterozygous familial hypercholesterolemia (HeFH), the reduction was 63.5% (Fig. 3). The general magnitude of these LDL-C reductions was sustained over 78 weeks of follow-up. In those with or without HeFH, the composite MACE event rate was 2.3% over this period relative to rates of 5.5% and 5.1% for these two groups, respectively, on statin therapy alone. The HR of 0.438 for the combined groups translates into a 56% risk reduction.
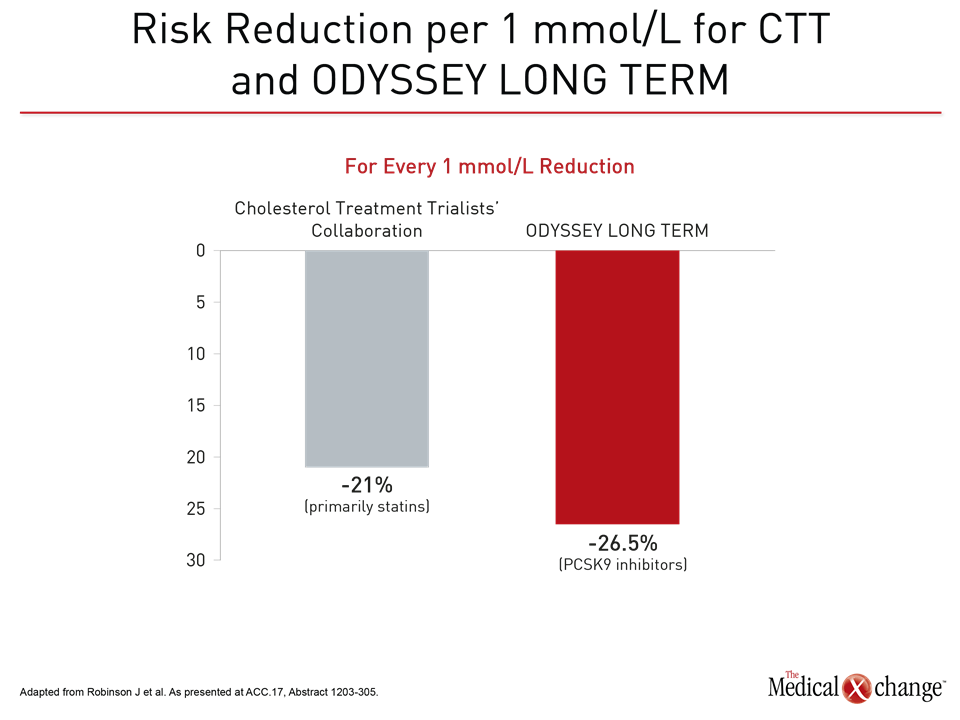
As observed in previous lipid-lowering studies, the reductions in LDL-C and reductions in CV risk were correlated. In ODYSSEY LONG TERM, each 1 mmol/L reduction in LDL was associated with a 26.5% reduction in the composite MACE endpoint.
Risk and Lipid Reductions Correlated
“In comparison, the Cholesterol Treatment Trialists’ (CTT) meta-analysis observed a 21% reduction in MACE per 1.0 mmol/L reduction in LDL-C,” reported Dr. Jennifer G. Robinson, Professor, Division of Cardiology, University of Iowa Hospitals and Clinics, Iowa City (Fig. 4). Presenting the ODYSSEY LONG TERM data at the ACC meeting, Dr. Robinson noted that the average LDL-C on alirocumab was 1.16 mmol/L in ASCVD patients with or without HeFH, and 1.11 mmol/L in those without HeFH. The end-of-study LDL-C among patients in the active treatment arm of FOURIER was about the same. These average levels are below those reached even with high-intensity statins, demonstrating that further reductions provide further CV protection.
“In the CTT meta-analysis, the CV risk reduction was 21% for each 1 mmol/L reduction in LDL-C. In ODYSSEY LONG TERM, the incremental reduction was 26.5%.”
As demonstrated in an open-label extension study of ODYSSEY LONG TERM presented by Dr. Robert Dufour, Institut de Recherches Cliniques de Montreal, Quebec, high doses of PCSK9 inhibitors are not required to reach these low LDL-C levels. After a washout period following their participation in ODYSSEY LONG TERM, 214 HeFH patients were initiated on 75 mg of alirocumab every 2 weeks. Physicians were permitted to adjust the dose after 8 weeks at their discretion. At the most recent analysis, only 48% had been moved to a dose of 150 mg every 2 weeks.
“Alirocumab dose titration by clinical judgment in a real world setting is feasible and effective.”
Noting that the average LDL-C reduction in this study was 52.6%, Dr. Dufour concluded that alirocumab dose titration by clinical judgment “in a real world setting” is feasible and effective.
PCSK9 Inhibitors Well Tolerated
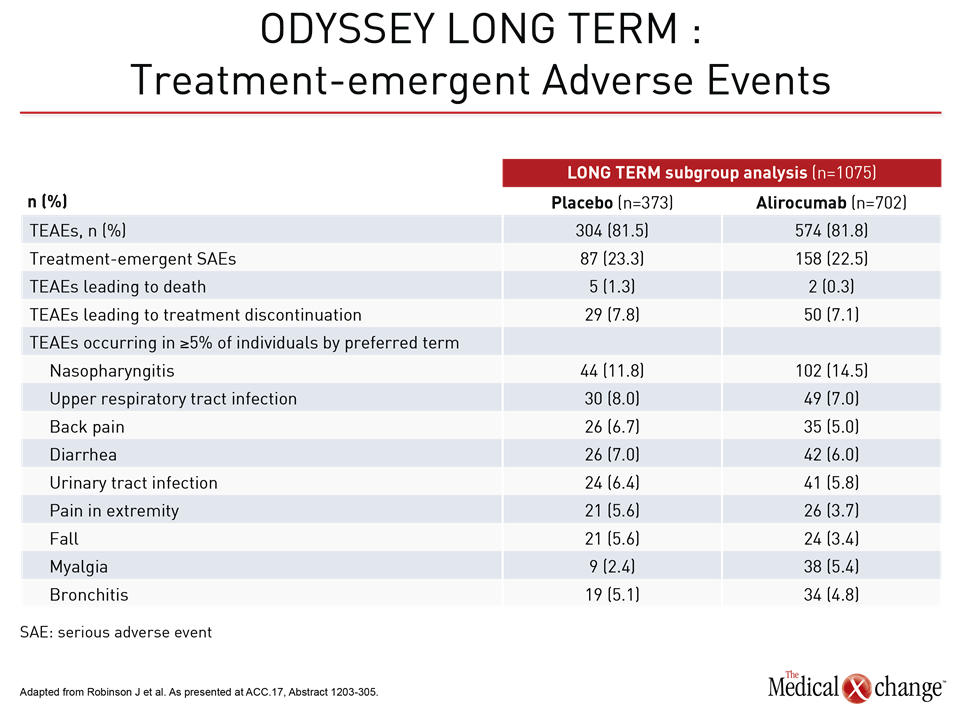
In ODYSSEY LONG TERM and FOURIER, the only consistently observed adverse event has been injection site reactions. In the ODYSSEY LONG TERM data presented by Dr. Robinson, the rate of treatment-emergent adverse events was numerically lower on alirocumab than placebo (22.5% vs. 23.3%) (Table 1). In FOURIER, no significant differences were seen in “overall rates of adverse events, serious adverse events, or adverse events thought to be related to the study agent,” according to the principle investigator, Dr. Marc Sabatine, Professor of Cardiovascular Medicine, Brigham and Women’s Hospital, Boston, Massachusetts.
“No significant differences were seen in overall rates of adverse events, serious adverse events, or adverse events thought to be related to the study agent.”
Importantly, there continues to be no signal of an adverse neurocognitive effect even among those who achieve LDL-C levels <1 mmol/L in either the ODYSSEY program with alirocumab or the clinical trials with evolocumab. A substudy of FOURIER, called EBBINGHAUS, performed intensive neurocognitive analyses on 1,974 patients from FOURIER and found no significant differences in any measure relative to placebo, according to Dr. Robert P. Giugliano, Professor of Cardiovascular Medicine, Brigham and Women’s Hospital. He reported that this was true even when evaluating a subgroup of patients who reached LDL-C levels <0.65 mmol/L. The median follow-up for changes in neurocognitive performance in this substudy was approximately 19 months.
“PCSK9 inhibitors relative to placebo were not only associated with a neutral relationship to serious adverse events (OR 0.98) but a greater than 40% reduction in all-cause mortality (OR 0.54).”
In a meta-analysis of 33 PCSK9 trials with 16,721 patients presented by Dr. Aris Karatasakis, University of Texas Southwestern Medical Center, Dallas, PCSK9 inhibitors relative to placebo were not only associated with a neutral relationship to serious adverse events (OR 0.98) but a greater than 40% reduction in all-cause mortality (OR 0.54; P=0.023). He indicated that mortality data, often considered the ultimate test of clinical benefit from treatment for a life-threatening disease process, provide a compelling rationale for offering agents in this class to patients at high risk of CV events.
Conclusion
PCSK9 inhibitors were approved for clinical use almost 2 years ago on the basis of their ability to provide improvements in LDL-C not attainable on statins alone. There is now proof that the correlation between LDL-C reductions achieved with PCSK9inhibitors and the subsequent reduction in CV risk is at least as robust as that observed with statins. For high-risk patients with persistently elevated LDL-C levels on statins alone, PCSK9 inhibitors provide an opportunity to achieve CV risk reductions not previously possible. However, treatment targets may also now be redefined. Evidence from the PCSK9 inhibitor trials indicate incremental reductions in CV risk continue to accrue with LDL-C lowering to levels of at least 1.0 mmol/L. Follow up now measured in years has provided evidence that these agents are safe and well tolerated.