Respirology
American Thoracic Society (ATS) International Conference 2017
Major Study Documents Additional Benefits for Anti-IL-5 Therapies in Severe Asthma
Washington, DC – New data from phase 3 trials with inhibitors of the interleukin-5 (IL-5) pathway have expanded information about the role of blocking eosinophil-driven inflammation to treat severe asthma. The data were presented at the 2017 ATS Conference. In one, conducted in patients with severe asthma, an experimental monoclonal antibody (MAb) targeting the IL-5 receptor (IL-5R) was associated with reduced exacerbation and use of oral corticosteroids (OCS). In studies with two commercially available MAbs that bind to IL-5, one showed that nearly one-third of patients entered into a phase 3 trial would also have been eligible for a biologic indicated for allergic asthma, and the other confirmed an improvement in health status from the patient’s perspective.
Benefits of Anti-IL-5 Therapies Broaden
Therapies targeted at interleukin-5 (IL-5), which mediates eosinophil maturation and proliferation, are poised to play a larger role in the treatment of severe airway diseases based on new data from phase 3 trials. In one, called ZONDA, benralizumab, a MAb that targets IL-5R, was associated with a 75% reduction (P<0.001) in oral corticosteroid (OCS) use. At the same time, the two benralizumab study regimens reduced annual exacerbation rates by 55% to 70% relative to placebo. In data derived from the reslizumab BREATH phase 3 program, benefit from IL-5 inhibition was observed in the 31% of patients who were also eligible for omalizumab, a biologic that targets immunoglobulin E (IgE). In a phase 3 mepolizumab trial, treatment benefit correlated with baseline eosinophil count.
ZONDA Trial: OCS Dosage Reduction
The Canadian-led ZONDA trial “was the largest study conducted to date of the OCS-sparing effect of a biologic for prednisone-dependent patients with asthma,” reported the principal investigator Dr. Parameswaran Nair, McMaster University and St. Joseph’s Healthcare, Hamilton, Ontario. “More than 50% of eligible patients were able to stop OCS completely.”
“ZONDA was the largest study conducted to date of the oral corticosteroid-sparing effect of a biologic for prednisone-dependent patients.”
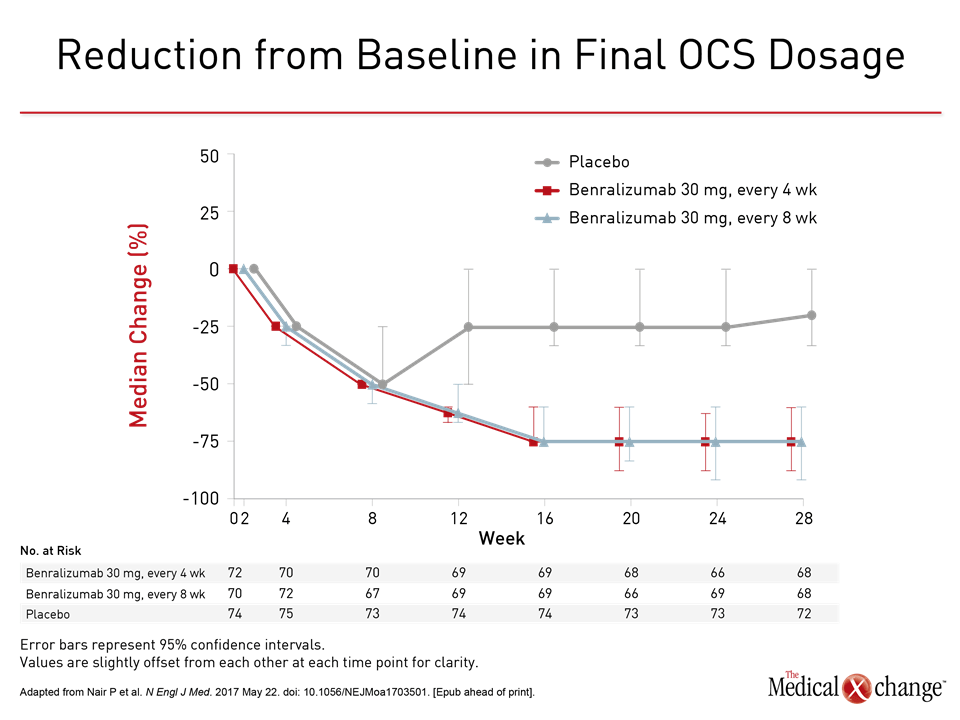
In the ZONDA trial, 220 patients who were taking OCS for at least 6 months and had a blood eosinophil count of ≥150 cells/mm3 were randomized in 12 countries to benralizumab at a dose of 30 mg administered subcutaneously (SC) every 4 weeks, benralizumab administered SC every 8 weeks, or placebo. Patients remained on their standard prescribed standard asthma medications. The primary endpoint was change in OCS dose from baseline to week 28.
“More than 50% of eligible patients were able to stop oral corticosteroids completely.”
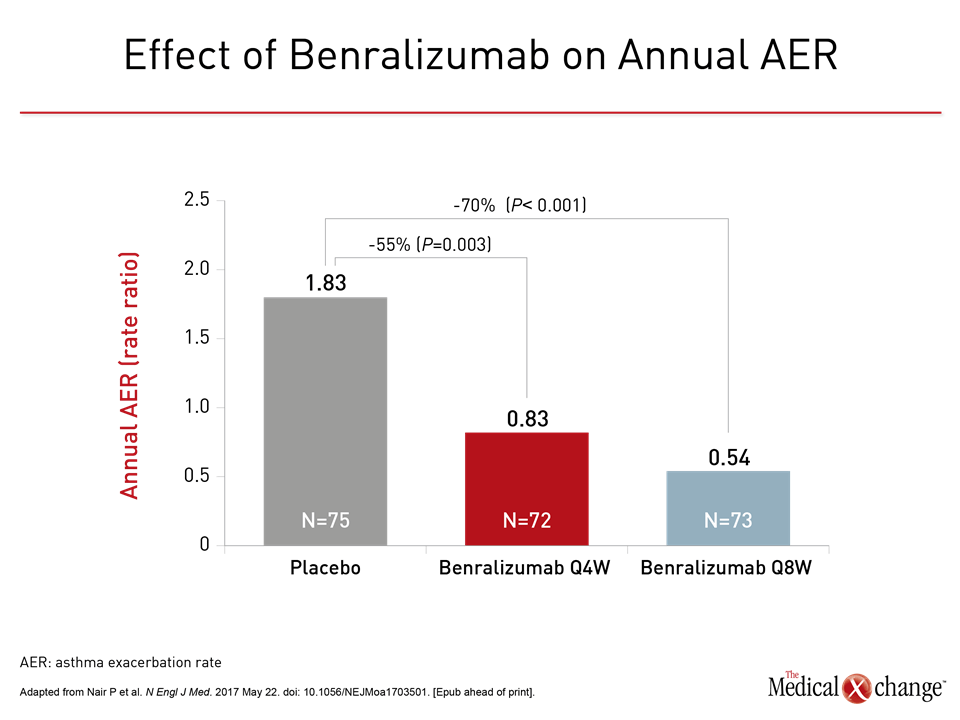
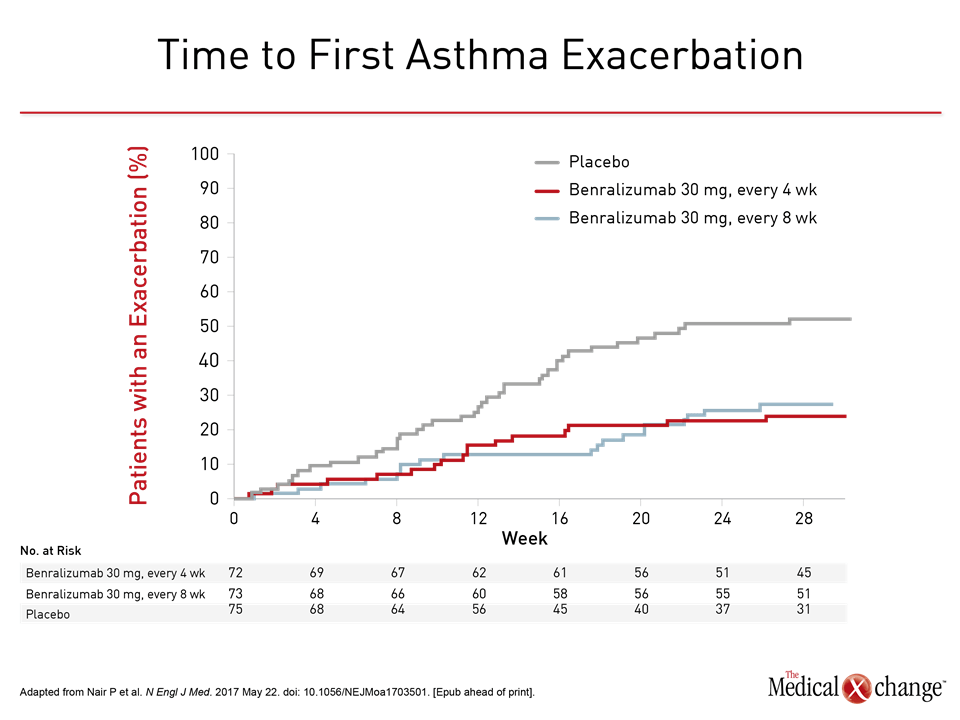
The median OCS dose was reduced 25% from baseline in the placebo group but 75% (P<0.001) in both groups receiving benralizumab (Fig. 1). OCS were stopped completely in eligible patients by end of study in 19% of those randomized to placebo versus 56% and 52%, respectively, of those in the every-4- and every-8-week regimens of benralizumab. The odds ratio (OR) for achieving a reduction in OCS on benralizumab versus placebo was approximately 4.1-times higher (P<0.001) for both active treatment arms. In addition to the reduction in OCS, patients randomized to either benralizumab arm had a relative protection from exacerbations. Lower odds of having at least one exacerbation versus placebo were observed in the study (OR for the every-4-week regimen vs. placebo: 0.32; P=0.001 and OR for the every-8-week regimen vs. placebo: 0.28; P<0.001), resulting in a longer time to the first exacerbation than placebo. When annualized, the relative reductions in exacerbations for these two groups were 55% (OR 0.45; P=0.003) and 70% (OR 0.3; P<0.001), respectively (Fig. 2). Rates of adverse events in the three groups were generally comparable. The numerically higher rate of events in the placebo group was attributed to the higher rate of worsening asthma. None of the most common adverse events, including nasopharyngitis and bronchitis, occurred more frequently on benralizumab than on placebo. The data from this trial “demonstrate that maintenance OCS could be reduced while asthma control was maintained,” Dr. Nair concluded. It is unclear whether benralizumab will be more effective than the existing anti-IL-5 therapies, which inhibit IL-5 rather than its receptor. However, Dr. Nair suggested that targeting the receptor rather than the cytokine has potential advantages, including greater depletion of luminal eosinophils that drive the inflammatory response.
Anti-IL-5 Benefit in Phenotype Crossover
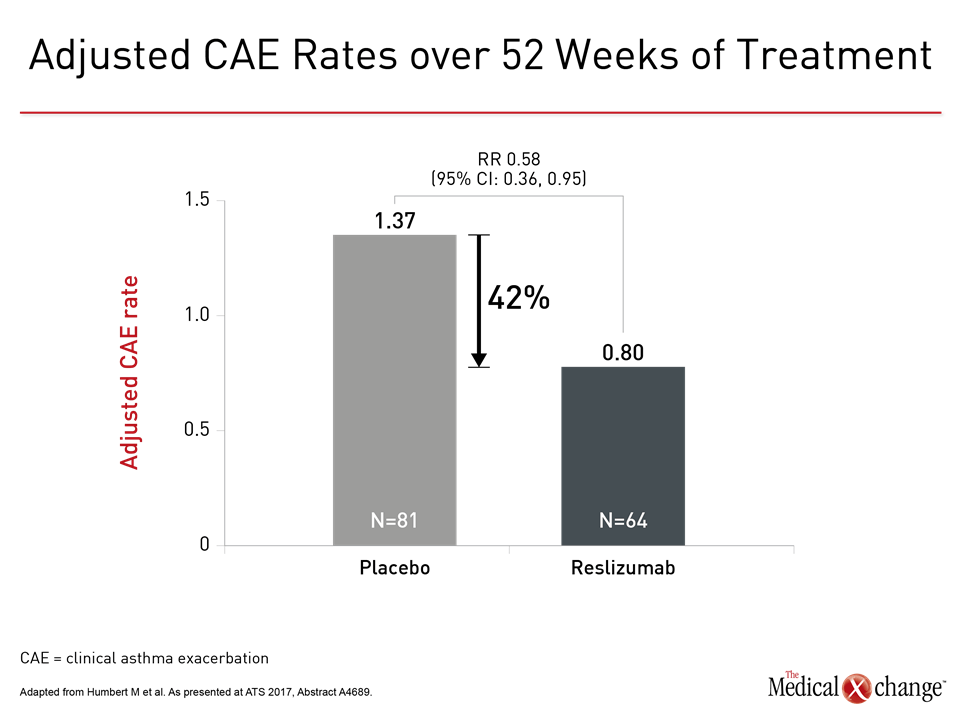
In an analysis of 464 patients with severe asthma who participated in the phase 3 BREATH program, which generated much of the data for licensing of reslizumab, 146 (31%) would also have been eligible for omalizumab, the anti-IgE MAb. The large crossover was not unexpected but a post-hoc analysis indicates that benefits in omalizumab-eligible patients were substantial. In the omalizumab-eligible subgroup, the anti-IL-5 therapy produced a 42% reduction in exacerbation rates relative to placebo. In this group, reslizumab was also associated with improvements in lung function as assessed with FEV1 and asthma symptoms as assessed with the six-item Asthma Control Questionnaire (ACQ-6). In this group, “the efficacy of reslizumab was consistent with that seen in the overall study and supports a clinically-meaningful benefit of reslizumab in asthma patients eligible for treatment with either biologic,” reported the principal investigator Dr. Marc Humbert, Chief of Respiratory Medicine, Hôpital Bicètre, Paris, France. He suggested more data are needed to understand selection criteria for patients eligible for both anti-IL-5 and anti-IgE biologics. In the phase 3 MUSCA study, response rates to mepolizumab corresponded to baseline eosinophils. In particular, there was a significantly lower improvement in FEV1 and in the 5-item ACQ score (ACQ-5) among those with the lowest baseline level (<150 cells/mcL). “Treatment effects for lung function and asthma control generally increased with baseline blood eosinophil count,” reported Dr. Jennifer L. Trevor, Head of the Severe Asthma Clinic, University of Alabama, Birmingham. In this sub analysis of the MUSCA trial, 551 patients were stratified by baseline eosinophil count. According to Dr. Trevor, “these data support the relevance of blood eosinophil thresholds” in selecting patients for anti-IL5 biologics. Additional Slides Figures 3 (Fig. 3) and 4 (Fig. 4).