Cardiology
European Society of Cardiology (ESC) Congress 2017
Progress in Isolating the Mechanisms of New Standards in Heart Failure Therapy
Barcelona – New information presented at the ESC Congress 2017 has provided further insight into the relative contribution of newly added standard therapies for heart failure (HF). According to the most recent HF guidelines from the Canadian Cardiovascular Society (CCS) (Howlett JG et al. Can J Cardiol 2016;32:296-310), the new standard agents, ivabradine and sacubitril/valsartan, are to be considered routinely in HF patients with left ventricular dysfunction progressing after receiving a previous standard triple therapy. Data presented here provide additional insight into the contribution of these newer therapies to improved outcomes.
Expanding on Therapies for HF Management
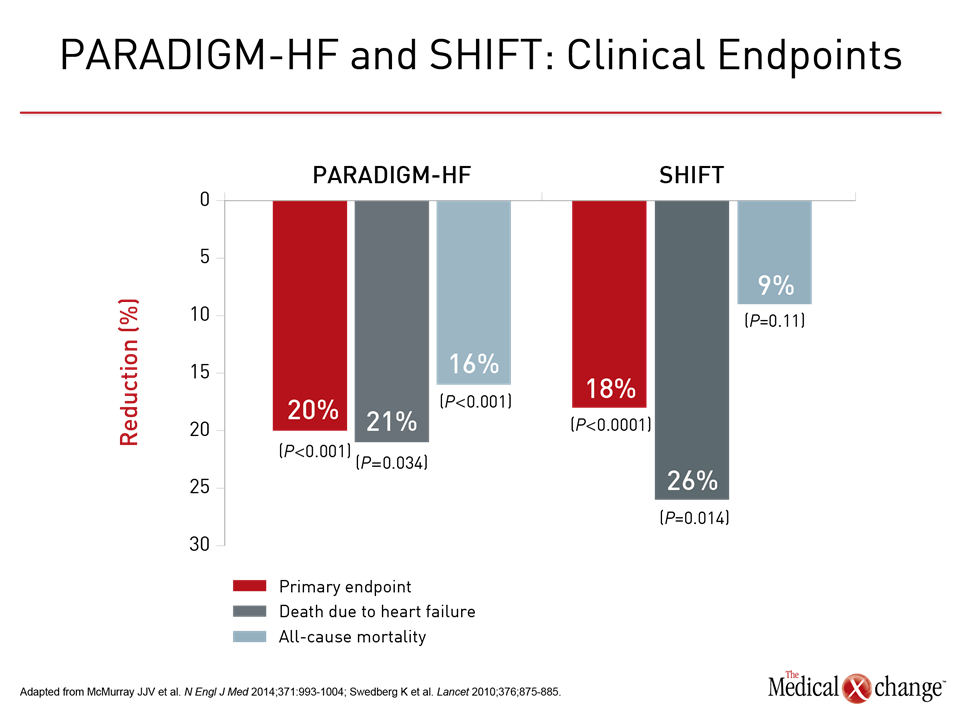
According to the 2016 CCS guidelines, the standard therapies for the management of HF have expanded. All standard therapies provide overall survival benefits or a reduction in deaths due to cardiovascular (CV) causes based on landmark multinational trials. ACE inhibitors, which were associated with a reduction in all-cause mortality, represent the first standard based on trials completed in the early 1990s. Subsequent additions included a beta blocker (BB) and a mineralocorticoid receptor antagonist (MRA) such as spironolactone. These three classes of drugs have been a standard since 1999. With the exception of subsequent studies suggesting that an angiotensin receptor blocker (ARB) can be substituted for the ACE inhibitor, nearly two decades passed before new standards were added. According to the CCS guidelines, the new additions, ivabradine and sacubitril/valsartan, are employed in patients progressing on triple therapy. New data derived from the registration trials with these two agents, which showed benefit in patients with reduced ejection fraction heart failure (HFrEF), expand insight into their clinical role. The role of the new standards, like the existing standards, was based on landmark trials. In the phase 3 SHIFT trial, ivabradine was associated with an 18% (P<0.0001) reduction in the composite primary outcome of CV death or hospital admission for worsening HF relative to placebo (Swedberg K et al. Lancet 2010;376:875-885). In addition to HFrEF, heart rate of ≥70 beats per minute was a SHIFT entry criterion. In the PARADIGM-HF trial, which was terminated early due to the clear advantage of the experimental arm, sacubitril/valsartan was associated with a 20% (P<0.001) reduction in the same endpoint as SHIFT but the advantage was relative to an active comparator, the ACE inhibitor enalapril (McMurray JJV et al. N Engl J Med 2014;371:993-1004). HFrEF was again an entry criterion but no heart rate parameters were specified. New data presented at this year’s ESC expand on the relative role and benefits of both these agents. In a SHIFT post-hoc analysis, the focus was on patients who were not taking BBs over the course of the study. These patients represented 10.5% of the study population. Overall, mortality was higher in patients who were not taking BB relative to those who were (27.3% vs. 15.7%; P=0.009). In the post-hoc analysis, the clinical benefits of ivabradine, including the CV mortality reduction, was found to be concentrated in this group.
Patients with HFrEF in sinus rhythm should receive a BB whenever possible, but, when this is not the case, ivabradine may reduce mortality in the absence of a BB.”
SHIFT Trial Data Support Beta-Blockers
These data from SHIFT are “consistent with the evidence that patients with HFrEF in sinus rhythm should receive a BB whenever possible, but, when this is not the case, ivabradine may reduce mortality in the absence of a BB,” reported Dr. John G. Cleland, Robertson Centre for Biostatistics, University of Glasgow, UK, who presented these findings (ESC 2017, Abstract 246). In the SHIFT study overall, the reduction in all-cause mortality in those who received ivabradine was 9% relative to placebo, a trend (P=0.11) that fell short of statistical significance. However, among the 685 (of the 6558 enrolled) who were not taking BBs, ivabradine was associated with a 30% (P=0.026) relative reduction in all cause mortality. For CV mortality, the relative reduction was 28% (P=0.05). According to Dr. Cleland, it is notable that those who did not receive a BB had higher heart rates in addition to higher mortality, suggesting rate control with either BB, ivabradine, or the combination is an important mediator of benefit.
“Compared to those treated with enalapril, patients treated with sacubitril/valsartan had less reduction over time in general HRQL.”
“We know from previous studies that adding ivabradine to BBs reduces HF hospitalizations but not risk of sudden death,” Dr. Cleland commented. “These data allow us to evaluate the effect of slowing heart rate by a mechanism independent of adrenergic receptor blockade.”
HRQL Data from PARADIGM-HF
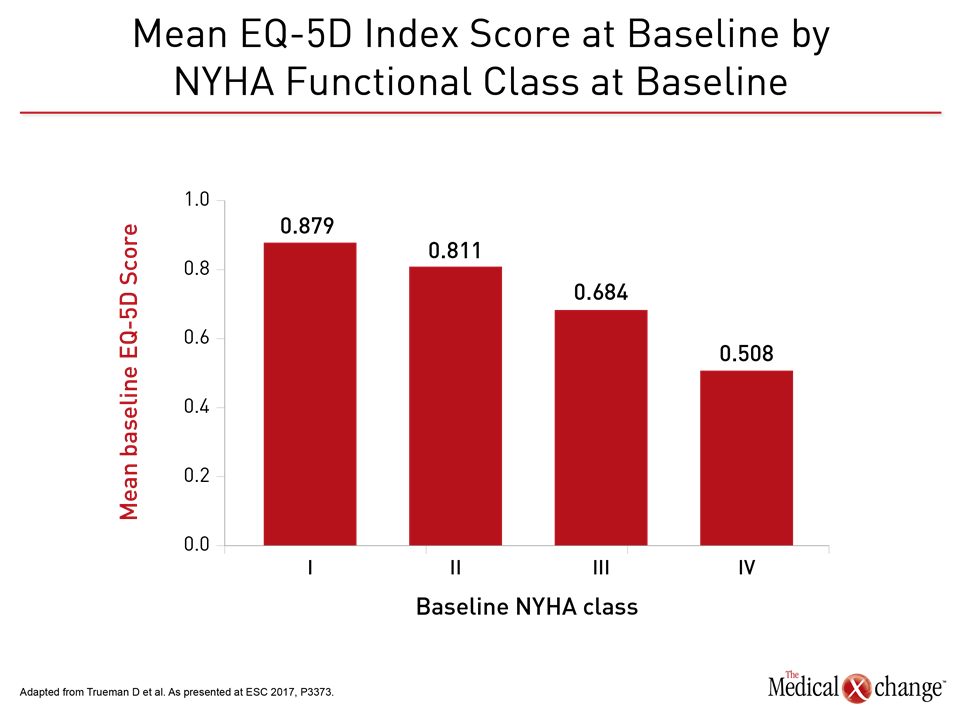
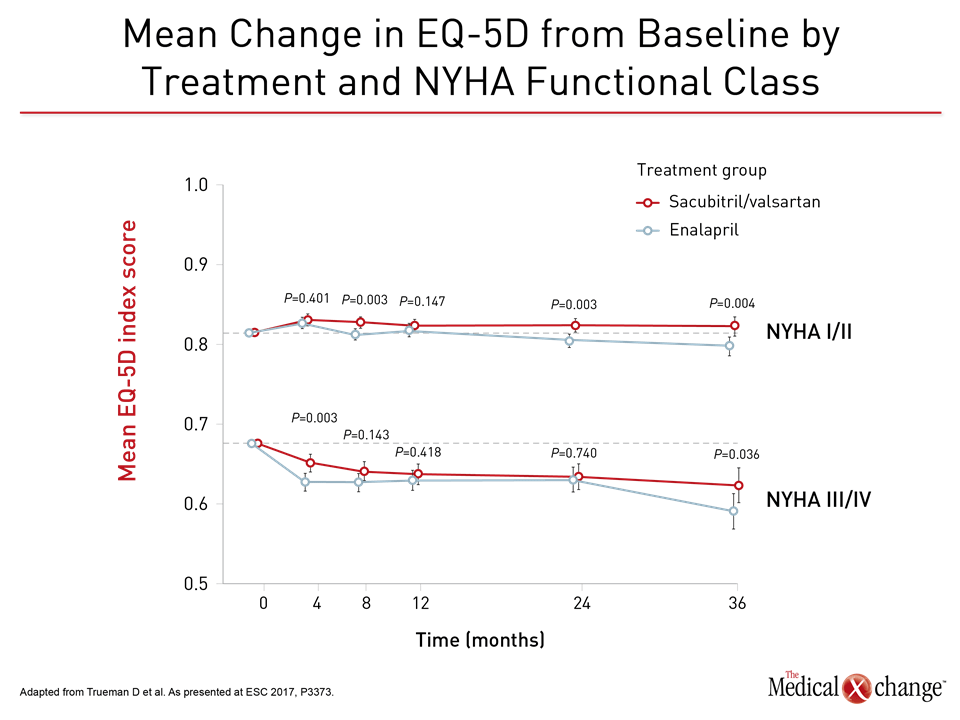
In two new sets of data from the PARADIGM-HF trial, one demonstrated that sacubitril/valsartan, in addition to protection against CV events, provided a statistically and clinically significant improvement in health-related quality of life (HRQL) relative to enalapril (ESC 2017, P3373). In the other, sacubitril/valsartan was associated with a reduction in anti-fibrotic biomarkers, which, in turn, correlated with lower CV events (ESC 2017, Abstract 248). The improvement in HRQL is particularly relevant to the CCS guidelines, which advocates switching patients with HFrEF from a renin-angiotensin-aldosterone system (RAAS) inhibitor such as an ACE inhibitor or ARB to sacubitril/valsartan on the basis of benefits observed in the PARADIGM-HF trial. “Compared to those treated with enalapril, patients treated with sacubitril/valsartan had less reduction over time in general HRQL, and the benefit was apparent irrespective of baseline NYHA class,” reported a team of PARADIGM-HF investigators that included Dr. Jean L. Rouleau, Professor of Medicine, University of Montreal, Quebec. In this analysis, the HRQL tool EQ-5D-3L, which measures the dimensions of mobility, usual activities, self-care, pain/discomfort, and anxiety/depression, was administered at baseline and then repeated at months 4, 8, 12, 24, and 36 of the study. At entry, almost 6000 patients were in NYHA Class II. Most of the remaining 2000 participants were in Class III or higher. As expected, there was an incremental decline in baseline EQ-5D-3L scores with each higher NYHA class (Fig. 1).Over time, there was a decline in HRQL in patients with Class I or II HF treated with enalapril, but this was largely prevented in those treated with sacubitril/valsartan (P=0.004 vs. enalapril). In those with more advanced disease, the rate of decline in HRQL was attenuated by sacubitril/valsartan relative to enalapril (P=0.036 vs. enalapril) (Fig. 2).
“HRQL is of utmost importance for patients with HF, especially those in the advanced stages of disease.”
“HRQL is of utmost importance for patients with HF, especially those in the advanced stages of disease,” commented Dr. Anique Ducharme, Director, Heart Failure Clinic, Montreal Heart Institute, Quebec. She noted that the EQ-5D-3L instrument is effective for “reflecting the limitations induced by HF in the daily living activities of a patient,” and that the attenuation in the decline in this measure of HRQL for sacubitril/valsartan relative to enalapril was seen in all NYHA classes. “Even though the numbers seem small, any improvement in HRQL for these patients is important.”
Anti-fibrotic Effect in PARADIGM-HF Substudy
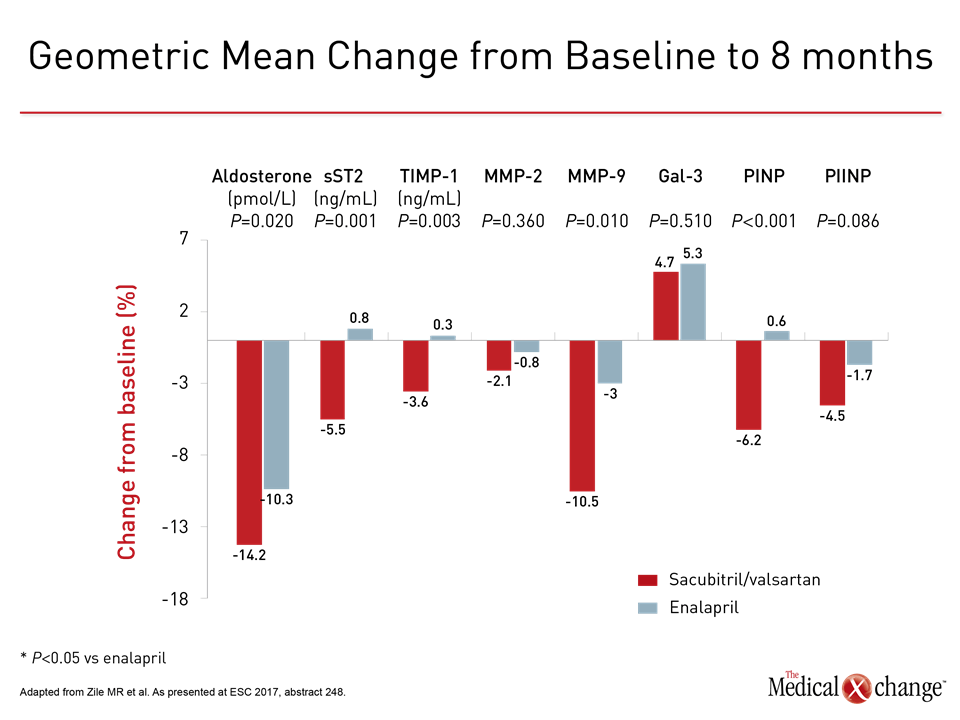
While these data confirm improvement in patients’ wellbeing with sacubitril/valsartan relative to enalapril as HF progresses, a second analysis from PARADIGM-HF identifies a potential mechanism by which this agent may prevent cardiac remodeling. In this study, extracellular matrix homeostasis biomarkers associated with profibrotic signaling were measured at baseline and then 8 months after randomization in a subset of approximately 1700 patients. Relative to referent values from healthy controls without HF, there were large increases in all eight markers of profibrotic signaling among participants in PARADIGM-HF, including aldosterone (68%), sST2 (92%), galectin-3 (88%), MMP-2 (97%), MMP-9 (66%), TIMP-1 (99%), PINP (65%), and PIIINP (78%).
“These data are consistent with the hypothesis that one mechanism of benefit from sacubitril/valsartan in HFrEF may be its ability to reduce progression of fibrosis.”
“We then looked at whether sacubitril/valsartan has an effect on these markers relative to enalapril over the course of treatment, and we found significant differences for five,” explained Dr. Michael Zile, Principal Investigator, Gazes Cardiac Research Institute, Medical University of South Carolina, Charleston (Fig. 3). “These data are consistent with the hypothesis that one mechanism of benefit from sacubitril/valsartan in HFrEF may be its ability to reduce progression of fibrosis.”
Profibrotic Biomarkers Correlate with Events
Supporting this hypothesis, increased expression from baseline in PARADIGM-HF of several of these biomarkers, including sST2, TIMP-1, and PIIINP, correlated with an increased risk of clinical events. “The two most significant relationships was a correlation between increases in sST2 and risk of the primary endpoint of death from CV causes or HF hospitalization and between increases in TIMP-1 and CV death,” Dr. Zile reported. These results point to a potential mechanism of action. Sacubitril/valsartan includes both valsartan, an ARB that like enalapril provides protection from RAAS activation, and sacubitril, which inhibits neprilysin. Neprilysin degrades natriuretic peptides, bradykinin, and other active substances participating in the neurohormonal overactivation that characterizes the vasoconstriction and maladaptive remodeling observed in HF. Although inhibitors of RAAS and neprilysin have been associated with protection from hypertrophy, published experimental evidence suggests that inhibition of both may have greater protection against cardiac fibrosis (von Leuder TG et al. Circ Heart Fail. 2015;8:71-8).
“I think the anti-fibrotic effect we saw [with sacubitril/valsartan] may be important both for the reduction in sudden cardiac death and the reduction in HF hospitalizations.”
“I think the anti-fibrotic effect we saw may be important both for the reduction in sudden cardiac death and the reduction in HF hospitalizations and HF-related death, because I think that a reduction in HF fibrosis would improve ventricular function, geometry, and the constitutive processes of the myocardium,” Dr. Zile explained. However, he cautioned that this perspective, while consistent with the substudy, is “all speculation” until more data can be shown to confirm that an anti-fibrotic effect from sacubitril/valsartan leads directly to improved outcomes. All of these data are consistent with the 2016 CCS guidelines. Triple therapy is recommended for NYHA Class I HF, but as patients progress to Class II or greater HFrEF, the guidelines reflect the results of the SHIFT and PARADIGM-HF trials. In patients with sinus rhythm and elevated heart rate (≥70 pbm), the guidelines recommend adding ivabradine to other standard therapies. In HFrEF patients in sinus rhythm with elevated natriuretic peptide, the guidelines recommend switching patients from their ACE inhibitor or ARB to sacubitril/valsartan while maintaining the other standard therapies. This switch is appropriate regardless of heart rate. These steps are associated with major improvements in objective outcomes. In PARADIGM-HF, in addition to the significant protection against the primary endpoint, sacubitril/valsartan was associated with a 16% reduction (P<0.001) for death from any cause. Although ivabradine was not associated with a reduction in all-cause mortality, it was associated with a 26% reduction (P=0.014) in deaths due to HF (Fig. 4). The recommended role of ivabradine and sacubitril/valsartan in the 2016 revised CCS guidelines largely mimic the entry criteria of SHIFT and PARADIGM-HF, respectively. In addition to HFrEF, these include an elevated heart rate in the case of ivabradine and elevated B-type natriuretic peptide (BNP) or NT-pro-BNP in the case of sacubitril/valsartan. The data presented at the ESC Congress 2017 support these recommendations while providing additional insight about the specific contributions these agents are making to improved outcomes.
Conclusion
Once initiated, HF is typically a progressive and terminal disease. The HF therapy of a RAAS inhibitor, BB, and MRA have long been a standard combination to delay this progression. Two additional standard therapies were identified in the revised 2016 revised CCS guidelines. The addition of these agents, ivabradine and sacubitril/valsartan, is based on survival benefit and improved morbidity in the phase 3 trials. New data generated from these trials provide additional insight about the types of benefits expected from these agents and the potential mechanisms of action. The data are consistent with the routine application of these now standard treatments in appropriate candidates to delay HF progression.