Respirology
European Respiratory Society (ERS) International Congress 2017
Stepping Up Therapy in COPD: New Lessons from Large Trials
Milan, Italy – Newly modified treatment guidelines in chronic obstructive pulmonary disease (COPD) were greatly strengthened by data presented at the 2017 ERS International Congress. In the guidelines, a long-acting muscarinic antagonist (LAMA) plus a long-acting beta agonist (LABA) were identified as the preferred therapy in advancing COPD, displacing the traditional step of a LABA plus an inhaled corticosteroid (ICS). The guideline change is evidence-based. Trial data have shown that LAMA/LABA is associated with a lower risk of both exacerbations and adverse events, including pneumonia and oral candidiasis. Multiple sets of data at this year’s ERS substantiated this message.
Expanded Data Consolidate Guideline Change
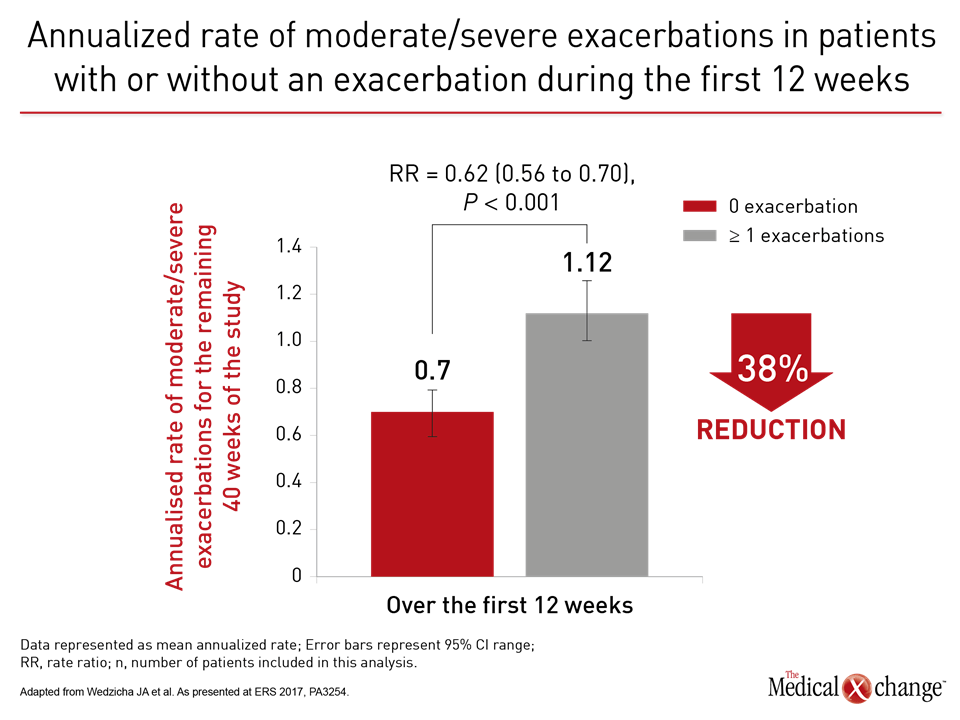
More than a year ago, the results of a landmark head-to-head comparison of treatment strategies in COPD showed convincingly that a LAMA/LABA combination reduced risk of exacerbations relative to a LABA/ICS combination (Wedzicha et al. N Engl J Med 2016;374:2222-2234). Based largely on this trial, called FLAME, the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines were revised earlier this year. The goal is to slow COPD by preventing or reducing exacerbations. Contributing to evidence that exacerbations drive disease progression, a substudy of FLAME showed this relationship independent of treatment assignment. In this substudy, presented at ERS by the principal investigator of FLAME, Dr. Jadwiga A. Wedzicha, National Heart and Lung Institute, Imperial College, London, UK, patients protected from exacerbations in the first 12 weeks of therapy had a 38% reduction in the annualized rate of subsequent moderate-to-severe exacerbations relative to those who were not. These data are wholly consistent with the premise of the GOLD guidelines. Early control of exacerbations prevents further exacerbations, a predictor of COPD progression. As stated by Dr. Wedzicha, these data reinforce the premise that “any prior exacerbation is a strong predictor for future moderate-to-severe exacerbations.” The data explain why FLAME data led to guideline changes.
Trial Data Alter COPD Guidelines
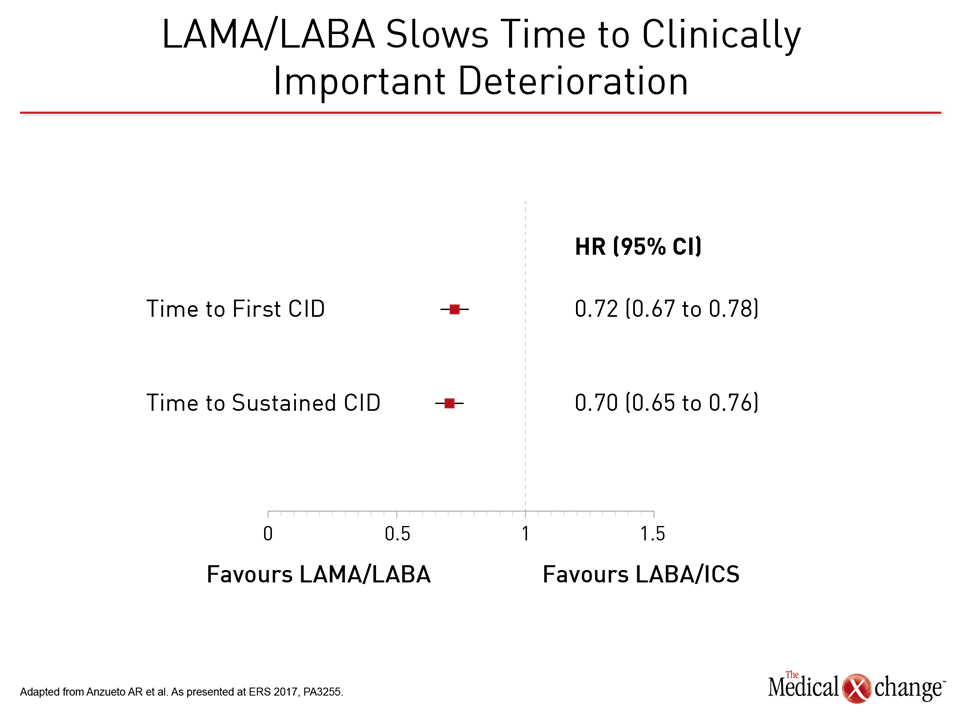
Relative to LABA/ICS (salmeterol plus fluticasone), the LAMA/LABA (glycopyrronium/indacaterol) was associated in FLAME with a 17% reduction (P<0.001) in the annual rate of moderate or severe COPD exacerbations. The LAMA/LABA combination was superior on several secondary endpoints, including prolonged time to the first moderate or severe exacerbation, with a 22% risk reduction (P<0.001). In addition, there were fewer adverse events on the LAMA/LABA, including pneumonia (3.2% vs. 4.8%; P=0.02). In the substudy presented at the ERS, the 1,369 patients without an exacerbation in the first 12 weeks of study were compared to the 1,697 with one or more exacerbations. The subsequent annualized rate of exacerbations was 0.70 in those with no exacerbations in the first 12 weeks versus 1.12 for those with one or more, translating into a 38% (P<0.001) reduction for those with good early control. Preventing exacerbations is consistent with GOLD guidelines. Each exacerbation increases risk for subsequent exacerbations (Fig. 1). A FLAME post-hoc analysis of clinically important deterioration (CID) generated similar results. In this study, CID was defined as any moderate-to-severe exacerbation or a ≥4 unit increase in the St. George’s Respiratory Questionnaire (SGRQ). Relative to the salmeterol/fluticasone arm and assessed on the basis of hazard ratio (HR), glycopyrronium/indacaterol delayed the time to first CID by 28% (HR 0.72; P<0.0001), according to Dr. Antonio Anzueto, Department of Pulmonary Diseases and Critical Care Medicine, University of Texas, San Antonio (Fig. 2).
CID: Relative Protection
When compared for sustained CID, defined as ≥100 mL decrease in FEV1 or ≥4 unit increase in SRGQ on two consecutive visits, the relative protection for the LAMA/LABA was similar, reducing risk by 30% (HR 0.70; P<0.0001). The protection was consistent across subgroups defined by gender, COPD severity, age, smoking status, and eosinophil count.
When compared for sustained CID, defined as ≥100 mL decrease in FEV1 or ≥4 unit increase in SGRQ on two consecutive visits, LAMA/LABA reduced risk by 30% relative to LABA/ICS.
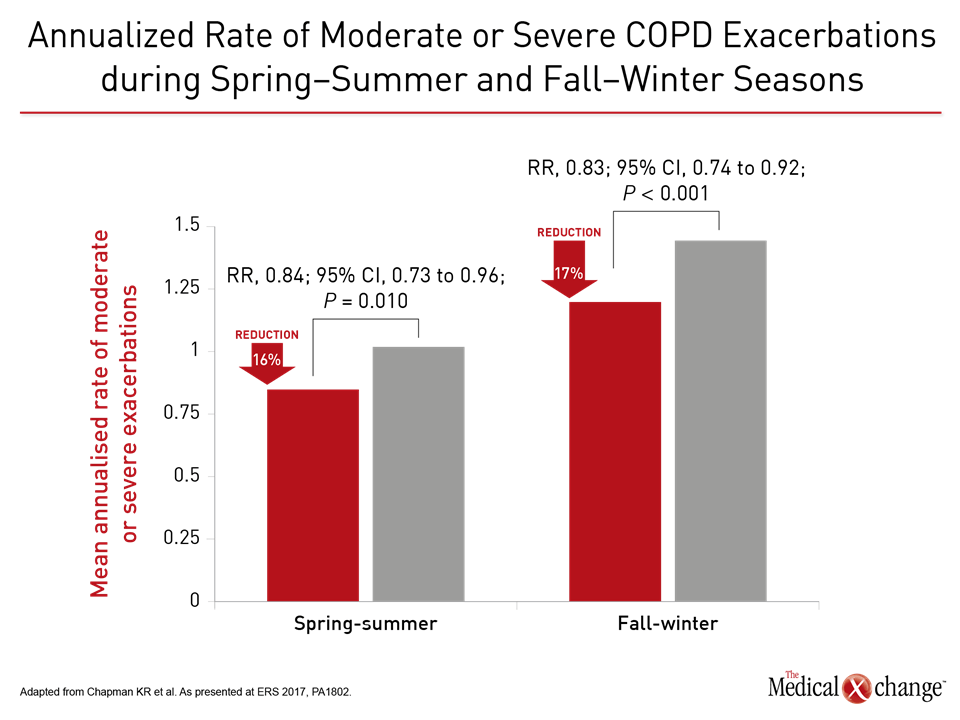
In another FLAME analysis, the relative protective effect of the LAMA/LABA versus LABA/ICS was demonstrated in fall/winter, when the annualized rates were 1.11 and 1.35, respectively (P<0.001). Although lower in spring/summer, the LAMA/LABA was still more effective (0.79 vs. 0.95; P=0.01) (Fig. 3). The lead author, Dr. Kenneth R. Chapman, Director of the Asthma and Airway Center, University Health Network, Toronto, suggested this is just one more set of data supporting the revised guidelines.
LAMA/LABA “should be considered as the preferred first-line treatment option in COPD patients at high risk of exacerbations.”
LAMA/LABA “should be considered as the preferred first-line treatment option in COPD patients at high risk of exacerbations,” Dr. Chapman stated.
GOLD Changes in Severity Groupings
In addition to reducing the frequency and severity of exacerbations, the goals of pharmacologic therapy in COPD are to reduce symptoms, improve exercise tolerance, and improve health status. According to GOLD guidelines, group B patients should be initiated on a dual bronchodilator when symptoms persist on a single bronchodilator, but the GOLD B population has also changed under the new GOLD guidelines. For the first time, spirometry readings are not used for severity groupings. Rather, these are entirely based on validated symptom measurement tools, such as the COPD Assessment Test (CAT).
With the elimination of lung function criteria, more patients are classified as GOLD A/B under the modified GOLD criteria for classifying severity.
On the basis of symptoms and exacerbation history without lung function studies, “more patients are classified as GOLD A/B,” explained Dr. Gary Ferguson, Research Director, St. John Providence Pulmonary and Critical Care Medicine, Farmington Hills, Michigan. This is relevant to patient care and when to consider the initiation of LAMA/LABA, but Dr. Ferguson used these new criteria to reevaluate the results from the TONADO registration trials with the LABA/LAMA tiotropium/olodaterol. In this trial with more than 5000 patients, the combination of tiotropium and olodaterol was associated with greater improvement in lung function and symptom control than either the LAMA or LABA alone (Buhl et al. Eur Respir J 2015;45:969-979).
Trial Reevaluated after GOLD Revision
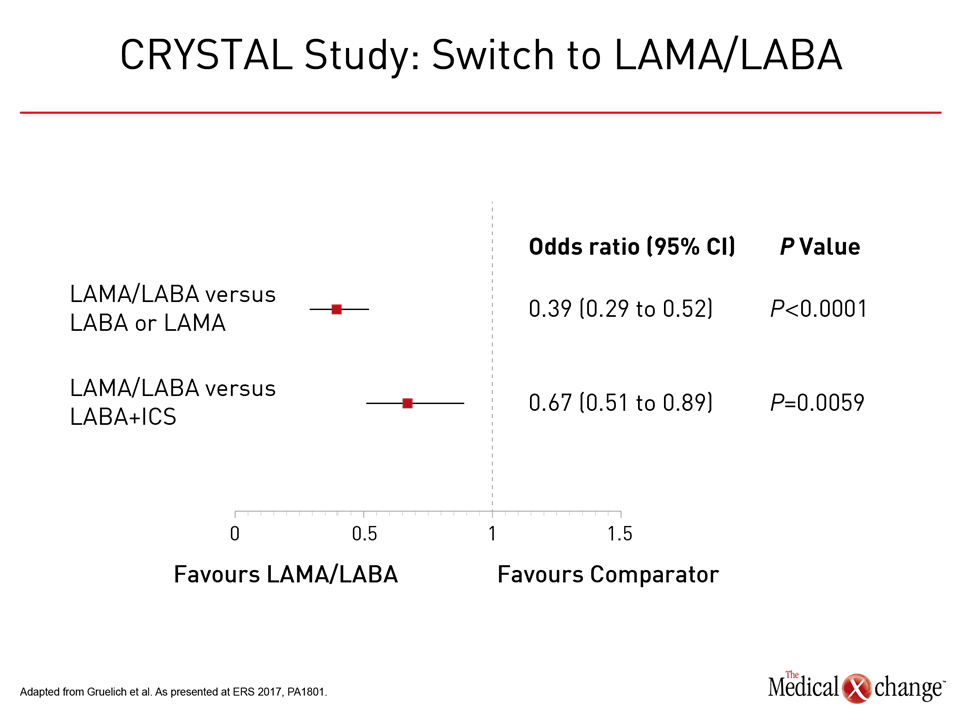
“When we reclassified patients in the TONADO studies using the 2017 GOLD criteria, we found an even greater impact on the two major endpoints, which were the transition dyspnea index (TDI) and the SGRQ,” Dr. Ferguson explained at this year’s ERS. The improved efficacy of the LAMA/LABA combination relative to either of its components, although not fundamentally different from that originally reported, is consistent with the new GOLD, although there was no ICS arm in the TONADO trial, which was conducted before the change in the guidelines. The advantage of switching from ICS/LABA to LAMA/LABA was part of the newly completed CRYSTAL study, which was also presented at the ERS. In this prospective multicenter randomized open-label study, CID was compared in patients switched a LAMA/LABA combination from those who remained on their baseline therapy, which could include LABA/ICS. Of the 2,159 patients, all of whom had moderate COPD, 1,622 switched and 537 continued on their baseline treatment. About half were taking LABA/ICS at baseline. The CID endpoint was defined as any moderate-to-severe exacerbation, a ≥100 ml decrease in trough FEV1, a ≥1 point decrease in TDI, or a ≥0.4 point increase in the COPD questionnaire (CCQ). Comparisons were made after 12 weeks. “After a direct switch from LABA/ICS, LABA alone, or LAMA alone, glycopyrronium/indacaterol significantly reduced the risk of CID inpatients with moderate COPD,” reported Dr. Timm Greulich, Division of Pulmonary and Critical Care Medicine, University Hospital, Marburg, Germany.
CRYSTAL Trial: Real World Data
The odds ratio (OR) for CID in this “real world” comparison was reduced by more than 60% when the LAMA/LABA was compared to either LAMA or LABA monotherapy (OR 0.39, 95% CI 0.29, 0.52). Relative to LABA/ICS, the rate of CID was reduced by 33% (OR 0.67; 95% CI 0.51, 0.89) (Fig. 4). The relative advantage was consistent across modified definitions of CID, such as lung function measures without inclusion of the CCQ measure of health status. Subgroup analysis by various patient baseline characteristics was also consistent with overall results.
“The LAMA/LABA was associated with fewer respiratory-related adverse events including, but not limited to, the risk of pneumonia.”
In addition to more reliable protection from CID relative to LABA/ICS, a dual LAMA/LABA bronchodilator strategy is safer, according to yet another data analysis from FLAME presented here. In this, the goal was to compare strategies for respiratory-related adverse events, which included a composite of events such as respiratory tract infections, pneumonia, and bronchitis. According to Dr. Wedzicha, who presented these data, the incidence rates were 3.3% for glycopyrronium/indacaterol versus 5.1% for salmeterol/fluticasone (P=0.013). In addition, the LAMA/LABA was associated with delayed to time to first report of pneumonia (HR 0.64; P=0.009). “The LAMA/LABA was associated with fewer respiratory-related adverse events including, but not limited to, the risk of pneumonia previously reported,” Dr. Wedzicha reported.
Triple Therapy with ICS: Defining the Role
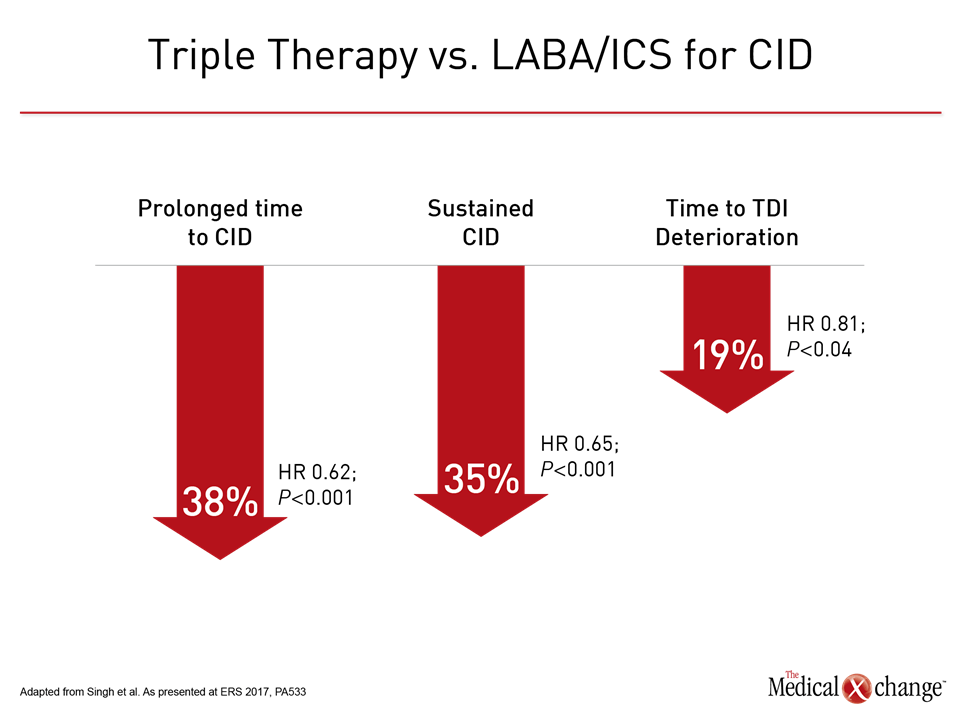
In patients deteriorating on LAMA/LABA, the revised GOLD guidelines suggest that the addition of an ICS may improve lung function and outcomes, but the guidelines note that there have been both positive trials, such as TRILOGY (Singh D et al. Lancet 2016;388:963-973), and negative trials (Aaron SD et al. Ann Intern Med 2007;146:545-555), and cautioned that more evidence is needed. While it is likely that many clinicians will consider this step in advancing COPD on the basis of the GOLD recommendation to individualize therapy, there were new data from the TRILOGY trial presented at the ERS to encourage triple therapy in advanced disease. Specifically, in the newly completed post-hoc analysis of TRILOGY, the triple therapy of the LAMA glycopyrronium, the LABA formoterol, and the ICS beclomethasone was compared to the combined LABA and ICS components for CID defined as any moderate-to-severe exacerbation, ≥100 ml decrease in trough FEV1, a ≥1 point decrease in TDI, and a ≥4 unit deterioration in SGRQ. Relative to LABA/ICS, triple therapy provided a nearly 40% (HR 0.62; P<0.001) protection against the time to first CID event, reported Dr. David Singh, Professor of clinical pharmacology and respiratory medicine, University of Manchester, UK. He reported that triple therapy also provided significant protection against components of CID, such as deterioration in TDI (Fig. 5). Although Dr. Singh concluded that these data “strengthen the role of this novel triple combination in COPD management,” one potential criticism is that the comparison was made prior to the change in GOLD guidelines, focusing on LABA/ICS, which is no longer the preferred therapy. Data are now needed to compare triple therapy to the new standard of LAMA/LABA.
Conclusion
In patients with COPD no longer adequately controlled on a single bronchodilator, the 2017 GOLD guidelines were changed to identify LAMA/LABA as the preferred strategy. New subgroup and post hoc analyses from the FLAME trial and other studies reinforce these recommendations. The advantage of a LAMA/LABA for reduction risk of exacerbations, a predictor of COPD progression, is accompanied by greater safety relative to LABA/ICS. These data should help accelerate practice change to the new standard in mild-to-moderate COPD.