Hematology
59th American Society of Hematology (ASH) Annual Meeting & Exposition
Myelofibrosis: Survival and Quality of Life Benefits from Early Intervention
Atlanta – Mature data from clinical trials in myelofibrosis with JAK inhibition have altered the treatment algorithm. According to expert analysis and a large new set of data presented at the 2017 ASH annual meeting, a JAK inhibitor should be considered the first-line option once patients become symptomatic. Alternative conventional therapies for controlling symptoms, such as hydroxyurea, are not equivalent because they are less effective and fail to address the underlying mechanism of disease.
JAK-STAT Signaling Drives Myelofibrosis
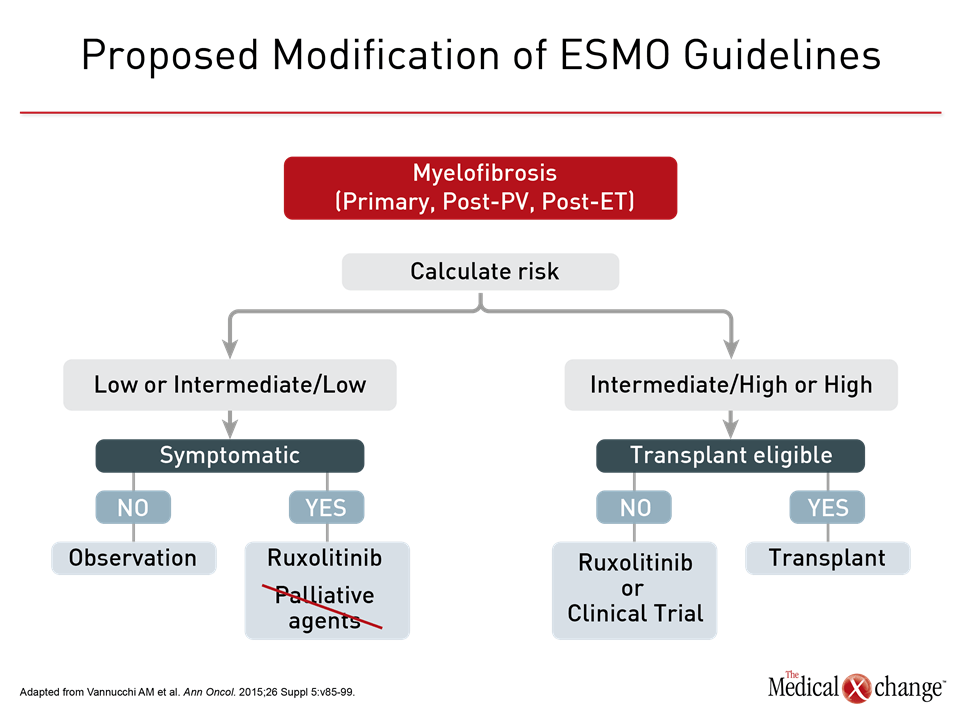
Whether due to a primary clonal myeloid expansion or neoplastic evolution from polycythemia vera or essential thrombocytopenia, myelofibrosis is largely driven by dysregulation of Janus kinase (JAK) mediated cytokine and growth factor signal transduction. The JAK2 V617F mutation is the most common underlying cause of this dysregulation, but other mutations, such as those of JAK2 exon 12 and calreticulin (CALR), also drive abnormal JAK-STAT signaling that underlies progression and symptom expression. This is critical to understanding the change in the approach to myelofibrosis. Although the JAK 1/2 inhibitor ruxolitinib has been listed as a first-line therapy in symptomatic low- and intermediate-risk myelofibrosis in many guidelines including, since 2015, those from the European Society of Medical Oncology (Vannucchi AM et al. Ann Oncol. 2015;26 Suppl 5:v85-v99), conventional agents were considered alternatives. This has changed, according to Dr. Claire Harrison, professor of myeloproliferative neoplasms at Guy’s and St. Thomas’ NHS Foundation Trust, London, UK (Fig. 1). Based on greater symptom relief and a survival benefit attributed to JAK inhibition, “I would very rarely use these [palliative] agents in my clinical practice,” said Dr. Harrison, who was referring to hydroxyurea and corticosteroids but included some off-label options, such as interferons. One of the world’s experts in myelofibrosis and a leader of an ASH Educational Session on this topic, Dr. Harrison emphasized that the advantages of JAK inhibition, once treatment is indicated, include better quality of life and extended survival.
JAK Inhibition Provides Survival Advantage
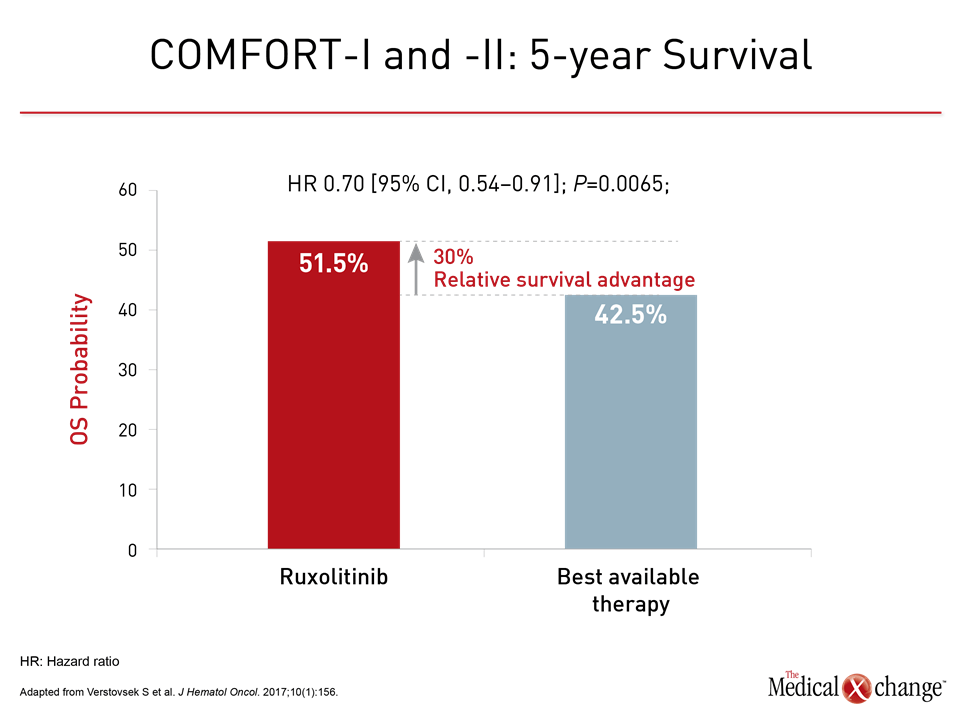
No therapy should be initiated in low risk myelofibrosis patients until symptoms develop, according to Dr. Harrison, but the phase 3 JAK inhibition data in symptomatic patients are compelling. In these trials, COMFORT I and COMFORT II, large reductions in symptoms relative to best available therapy (BAT) were reported at the time of publication. Subsequently, both studies associated ruxolitinib with a survival benefit despite the large crossover from BAT to ruxolitinib at the end of these trials. In a recent pooled phase 3 analysis cited byDr. Harrison, upfront ruxolitinib was associated with a 30% reduction in death.
“The survival benefit of ruxolitinib has now been added to its label, but it is not the primary reason, at least for myself in clinical practice, for initiating patients on this drug.”
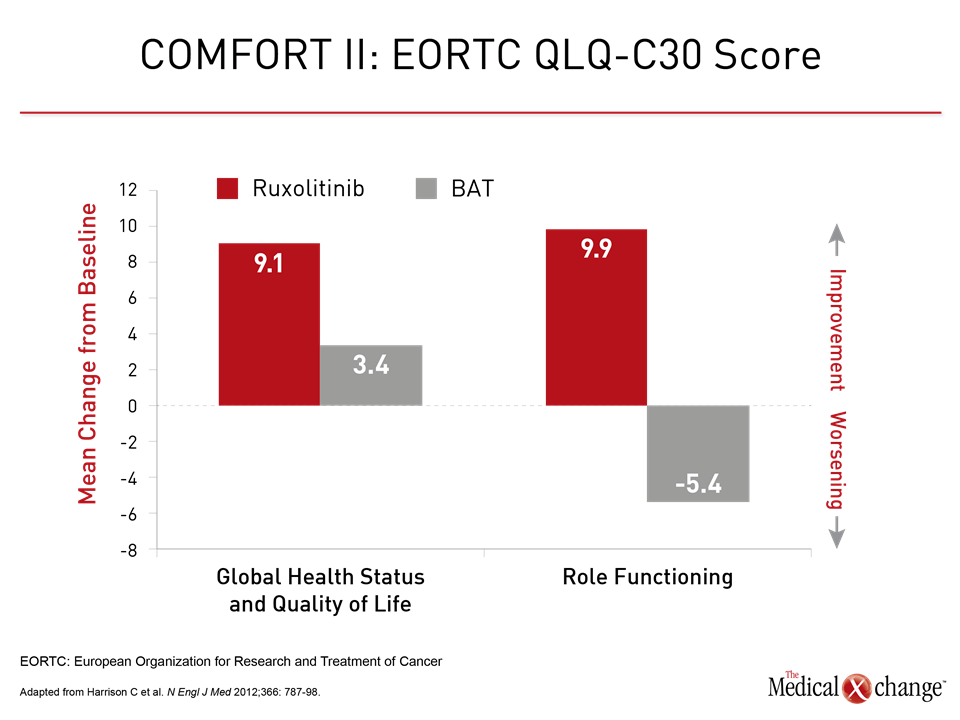
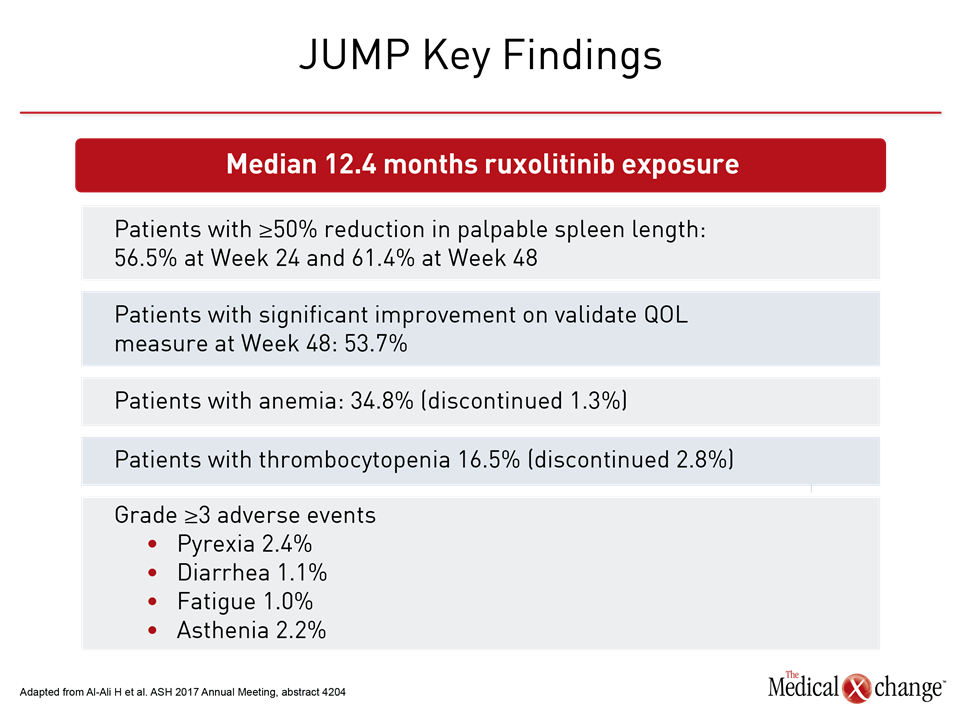
“The survival benefit of ruxolitinib has now been added to its label, but it is not the primary reason, at least for myself in clinical practice, for initiating patients on this drug,” Dr. Harrison reported. Rather, she emphasized that ruxolitinib provides a sustained reduction in the symptomatic burden of the disease (Fig. 2). Extending patient survival is important, but Dr. Harrison suggested that permitting patients to achieve an acceptable quality of life over the course of that survival also deserves emphasis. In the JUMP study, which was presented at this year’s ASH meeting, 61.4% of patients on ruxolitinib achieved at least a 50% reduction from baseline in palpable spleen length at week 48. In this study with 2,233 patients treated in 26 countries, which makes it one of the largest studies ever conducted in myelofibrosis, the improvements in splenomegaly were accompanied with reduction in characteristic constitutional symptoms such as fatigue, pruritus, arthralgias, and myalgias. In turn, the reduction in symptoms corresponded with improvements in validated quality of life instruments.
JUMP Study Enrolls Broad Population
“Consistent with previous studies, anemia and thrombocytopenia were the most common adverse events, but these rarely led to discontinuation of ruxolitinib,” reported Dr. Haifa Kathrin Al-Ali, Department of Hematology/Oncology, University Hospital, Halle, Germany. In fact, the findings are very similar to those reported in the two phase 3 COMFORT trials, but they confirm effects in a much broader population over a longer period of time. In her analysis, Dr. Harrison, who cited the JUMP trial data as well as the previously published phase 3 trials (for which she served as a principal investigator of COMFORT II) said that the ruxolitinib adverse event profile is manageable. Thrombocytopenia should be monitored but is not typically symptomatic. Anemia is more likely to be symptomatic, particularly in the first 12 to 16 weeks “but hemoglobin then begins to move back toward baseline.” She suggested supportive therapy, such as danazol or an erythropoietin-stimulating agent, could be helpful during this period. Upfront ruxolitinib should be initiated quickly once symptoms develop even in low risk patients because myelofibrosis is a rapidly progressive disease for which symptom control should not be the only consideration. Nearly 90% of patients with this disease die of myelofibrosis-related complications. Once symptoms develop, early and sustained inhibition of the JAK-STAT pathway offers the best opportunity of delaying end stage disease. “If you do decide to stop ruxolitinib for your patient, please be aware that patients who discontinue ruxolitinib have generally a very poor prognosis,” Dr. Harrison said.
Conclusion
Prior to the introduction of a JAK-STAT pathway inhibitor, the median survival for symptomatic myelofibrosis patients was an estimated six years. Early use of an agent targeted at this pathway has been shown to prolong survival relative to conventional therapies, but the often dramatic improvement in symptoms with a JAK inhibitor is also associated with meaningful improvements in QOL over the period of this extended survival.