Hematology
59th American Society of Hematology (ASH) Annual Meeting & Exposition
Polycythaemia Vera: JAK Inhibition after Hydroxyurea Resistance
Atlanta – Four-year results from a phase 3 trial confirm that JAK inhibition is effective for long-term control of polycythemia vera (PV) resistant to hydroxyurea. To date, the median duration response in those treated with the JAK inhibitor has still not been reached, according to updated data presented at the 2017 ASH annual meeting. Joined by data from two real-world studies presented here, the data expand the evidence that JAK inhibition is the appropriate next step after hydroxyurea, slowing important milestones of this disease process.
New Data Establish Sustained Benefit
PV, a progressive myeloproliferative neoplasm characterized by erythrocytosis, is associated with splenomegaly, pruritus, and fatigue. With rising hematocrit (HCT) and platelet counts, patients also face risk of thrombotic events. The phase 3 RESPONSE trial provided the basis of regulatory approval of the JAK inhibitor ruxolitinib for PV (Verstovsek S et al. Haematologica 2016;101:821-9). This was based on the advantage of this agent over best available therapy (BAT) at 80 weeks. The newly-reported data show that key measures of disease control are preserved at 208 weeks, validating protection from progression. “The response rates have been durable in both those initially randomized to ruxolitinib and those in the crossover group, while the safety profile of ruxolitinib after long-term treatment was consistent with that observed in the initial analysis,” reported Dr. Jean-Jacques Kiladjian, a consultant hematologist and head of the Clinical Investigation Center, Hôpital Saint-Louis, Paris, France.
“The response rates have been durable in both those initially randomized to ruxolitinib and those in the crossover group.”
In the multinational RESPONSE trial, 222 PV patients resistant or intolerant to hydroxyurea were randomized to the JAK 1/2 inhibitor ruxolitinib or BAT, which included a variety of investigator-selected agents, including hydroxyurea, or no therapy. Patients in the BAT arm were allowed to crossover to ruxolitinib 32 weeks after randomization. The limited options in the BAT arm and the decision to permit crossover at 32 weeks reflect the need for next-step therapy. Of the 112 patients randomized to BAT, 98 (87.5%) crossed over, making the relevant long-term comparisons between the groups initiated on ruxolitinib and those crossed over.
Primary Response to Ruxolitinib Sustained
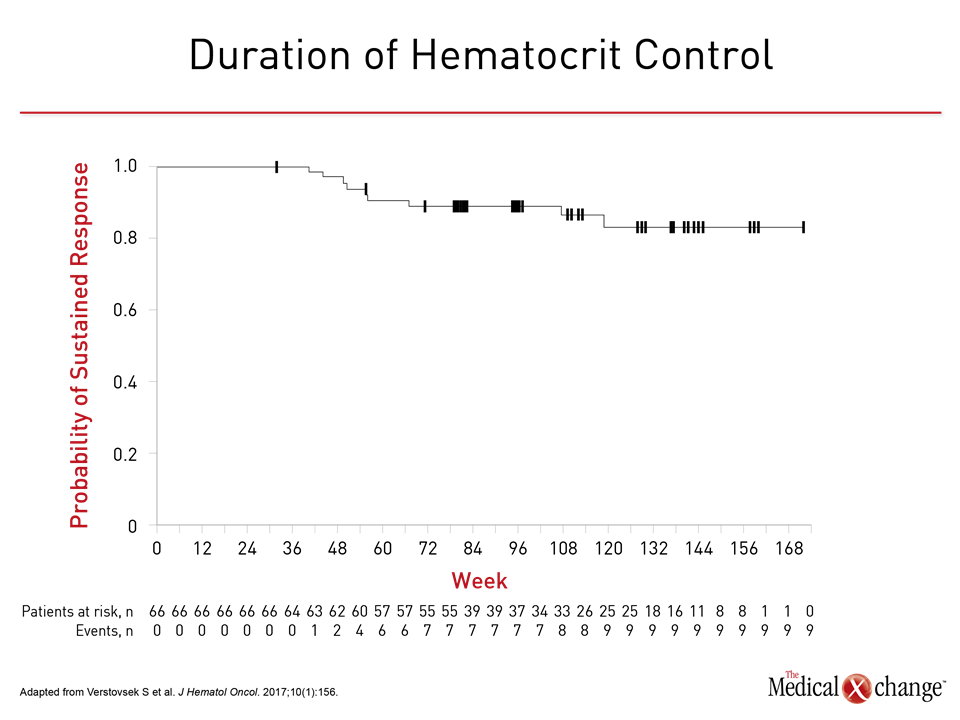
In the 80-week and the 208-week data, sustained benefit was seen in both groups for both the primary response (defined as phlebotomy-independent HCT control of <45% and ≥35% spleen volume reduction) and the clinicohematologic (CLHM) response, which, in addition to the primary response endpoints, included platelet (≤400 x 109/L) and white blood cell (≤10 x 109/L) control. At week 32, as reported in the published study, HCT control (60% vs. 18.8%), spleen response (40.0% vs. 0.9%) and primary response (22.7% vs. 0.9%) were all higher in the group randomized to ruxolitinib. When compared at 80 weeks, control as measured with HCT, platelets, and white blood cell counts were similar. The duration of HCT control was sustained over the course of the study (Fig. 1). In the 208-week data, 37% of patients initially randomized to ruxolitinib and 38% of those crossed over to ruxolitinib remained on treatment. Only six (24%) of the 25 who achieved a primary response and 21 (30%) of the 70 patients with a CLHM response have progressed so far. The durability of the primary response from week 108 to week 208 has also been sustained.
Median Responses Not Yet Reached
“The median duration of primary and CLHM responses have not yet been reached,” reported Dr. Kiladjian. He noted that initiating patients on ruxolitinib, related to the delay for crossover, has been associated with a numerical advantage for estimated 5-year overall survival (90.6% vs. 87.7%). The thromboembolic event rate, which was lower at week 32 in those randomized to ruxolitinib than BAT (1.8 vs. 8.2 per 100 patient years), has remained low in both groups. The findings are consistent with the mechanism of action. Essentially all PV is associated with mutations affecting JAK-STAT signaling. By inhibiting this pathway, ruxolitinib targets the underlying pathophysiology. The original data from the RESPONSE phase 3 trial, now expanded with 208-week data, has confirmed prolonged benefit once hydroxyurea fails.
Real-World Data Corroborate Benefit
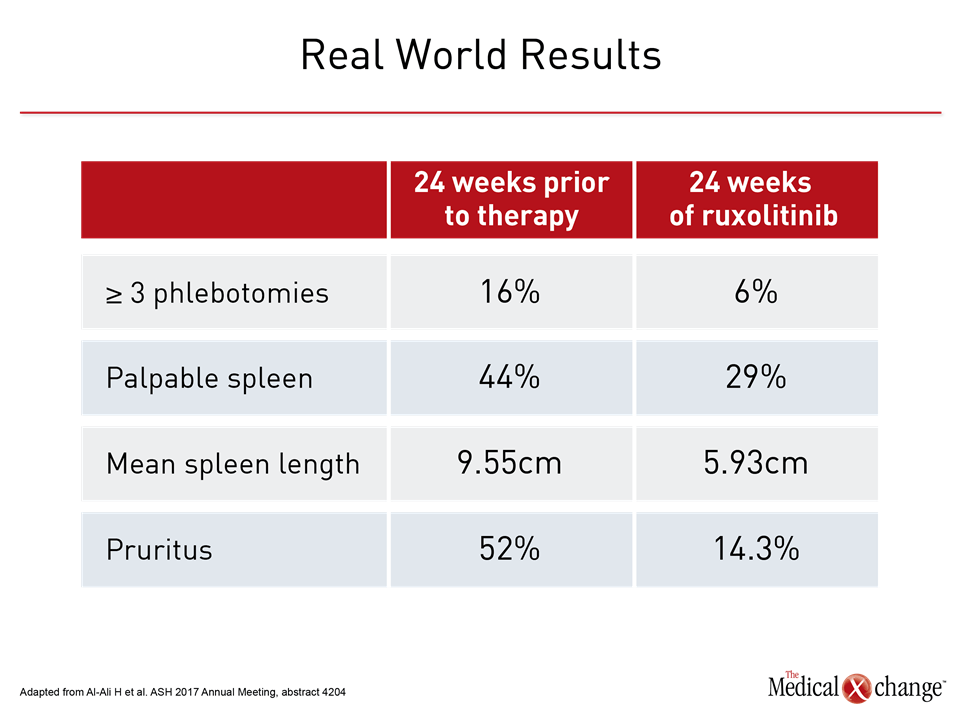
Two real-world experiences presented at this year’s ASH meeting generated comparable data. In one, 25 patients were evaluated at least 32 weeks after initiating ruxolitinib. The reductions in need for phlebotomy, spleen size, symptoms, HCT, and other measures of PV were “largely congruous with the positive results of the original RESPONSE trial,” reported Dr. Alexander Coltoff, Icahn School of Medicine at Mount Sinai, New York, NY (Table 1). Calling his study “the first real-world evaluation of ruxolitinib in the treatment of PV,” he added, “16% of the patients in our study discontinued ruxolitinib, similar to the 15.5% in the RESPONSE trial, indicating that the medication was largely well tolerated.” In a second multicenter, expanded-access phase 3b study, 75 patients were enrolled. Slightly more than half of patients were hydroxyurea intolerant and the remaining were resistant. By week 24, 52 patients (69%) had achieved HCT control (<45%), reported Dr. Timothy Devos, Department of Hematology, University Hospital, Leuven, Belgium. “At week 24 in this study, patients treated with ruxolitinib experienced benefits in terms of HCT control, hematologic remission, reduction in spleen size, and reduction in symptom score consistent with those seen in previous clinical trials of ruxolitinib in patients with PV,” Dr. Devos reported. He, like Dr. Coltoff, concluded that these data support this targeted therapy as a next step for patients who do not respond or are no longer responsive to hydroxyurea.
Conclusion
For patients with progressive PV, hydroxyurea has been the most common first-line cytoreductive therapy for the treatment of PV, but a substantial proportion of patients have an inadequate response at acceptably tolerated doses or lose response over time. In those no longer responsive to hydroxyurea, the RESPONSE trial established the JAK 1/2 inhibitor ruxolitinib as a next step. Expanded data have confirmed clinically meaningful and durable control of disease progression.