Respirology
American Thoracic Society (ATS) 2018 International Congress
In COPD, Large Percentage of Clinicians Ignore Evidence-based Guidelines
San Diego – According to current treatment recommendations for chronic obstructive pulmonary disease (COPD), inhaled corticosteroids (ICS) should be introduced only in those patients experiencing frequent exacerbations on dual bronchodilators. Several sets of data, including those presented at this year’s ATS International Conference, demonstrate that real-world practice is not consistent with this recommendation. Rather, ICS is commonly added to a bronchodilator despite the fact that a dual-bronchodilator regimen is the preferred therapy. In addition, many patients with COPD are being treated with ICS-containing triple therapy rather than with dual bronchodilators. Experts are searching for strategies to align care with evidence-based medicine, including both initiating the appropriate therapy and weaning patients from triple therapy to avoid the unwarranted risks and costs that guideline-directed care permits.
Same Trends in Canada as Reported Elsewhere
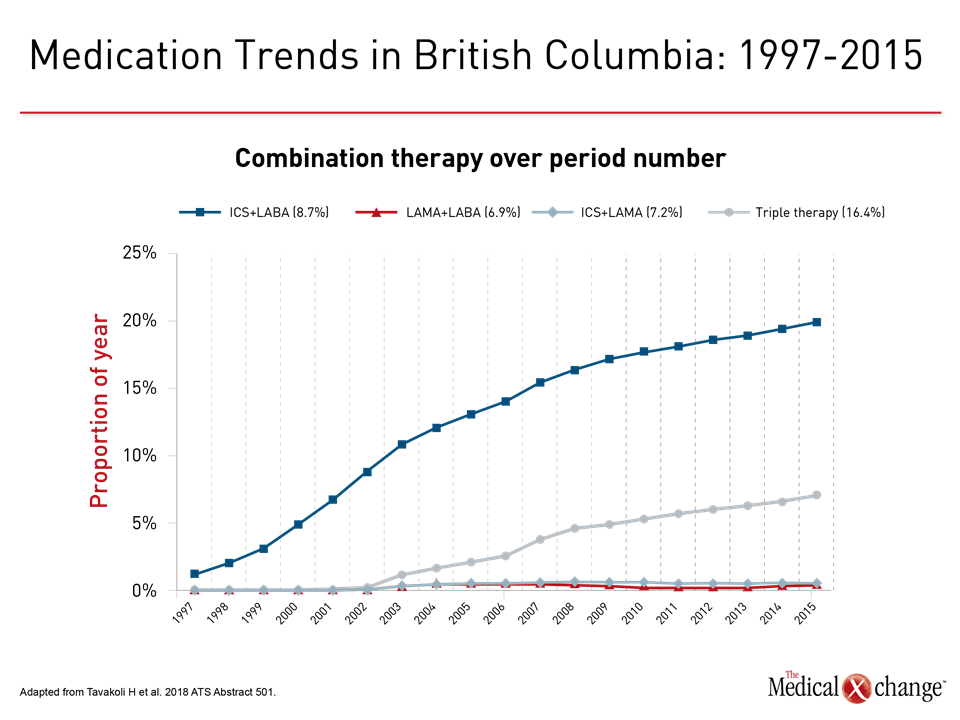
As reported in other countries, clinicians in Canada have not so far adjusted to guideline-mandated care of COPD, according to a study that evaluated use of inhaled medications by more than 176,000 patients in British Columbia. When combination therapy was standardized by dose, prescriptions for ICS plus a long-acting beta agonist (LABA) have been climbing steeply and steadily since the year 2000. The climb in ICS-containing triple therapy started later, but also involves a steep increase (Fig. 1). This is the opposite of what would be expected from evidence-based care.
“How can we improve patient care in the real world setting so that patients are treated in line with what our guidelines are suggesting?”
These data pose the question, “How can we improve patient care in the real world setting so that patients are treated in line with what our guidelines are suggesting,” commented the senior author of the study, Dr. Mohsen Sadatsafavi, Division of Respiratory Medicine, University of British Columbia, Vancouver.
Perhaps most surprising from the British Columbia data is that dual bronchodilator therapy, which is the guideline-based preferred first-line combination “is nowhere. It’s stuck to the floor,” Dr. Sadatsafavi said. Overall, the rise in ICS-containing combination therapies for COPD has been “dramatic.” Yet, according to the most recent Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidance document, these trends are going in the wrong direction.
LABA/LAMA Is Preferred First-Line Combination
According to GOLD, the preferred first-line combination in patients with COPD insufficiently controlled on a single bronchodilator is a LABA plus a long-acting muscarinic antagonist (LAMA). Dual bronchodilators are an evidence-based preference over ICS plus a bronchodilator on the basis of the multicenter randomized FLAME trial (Wedzicha JA et al. N Engl J Med. 2016;374:2222-34). In a head-to-head comparison, the LABA indacaterol plus the LAMA glycopyrronium provided greater protection against exacerbations than a combination of the LABA salmeterol and the ICS fluticasone.
Although the cut-off for the British Columbia data in 2015 occurred prior to publication of the most recent COPD management strategies, the steep rise in ICS-containing combinations, particularly triple therapy, is not consistent with previous GOLD recommendations that call for the addition of ICS to dual bronchodilators only in patients with frequent exacerbations. In an evaluation of a US database with almost 200,000 patients, 25% of all patients on maintenance therapy were taking triple therapy, but 75% of these patients had either mild or moderate COPD (Simeone JC et al. Int J Chron Obstruct Pulmon Dis. 2017;12:73-83).
“This study indicates that a substantial percentage of patients receiving triple therapy are GOLD grade 1 or 2,” the authors of the published study reported. One problem may be that clinicians are not even considering COPD grade when they prescribe inhaler therapy. In another finding from this study, “over half of patients [receiving triple therapy] did not receive the spirometric testing required to assess airflow limitation.”
Mixed Reasons for Triple Therapy
There may be several reasons why clinicians initiate patients with COPD on ICS-containing combinations. For ICS/LABA, clinicians may be unaware that LABA/LAMA is the evidence-based first choice over ICS/LABA on the basis of efficacy. For triple therapy, some clinicians might inaccurately believe that moving directly to high-intensity therapy will slow progression. According to GOLD, no treatment has been shown to be effective for slowing progression, yet there are numerous disadvantages of adding ICS to a bronchodilator for the treatment of COPD. Current international treatment recommendations are that triple therapy should be reserved only for high-risk patients still experiencing exacerbations while taking a dual bronchodilator.
“The long-term use of ICS is associated with several significant adverse events including pneumonia, mycobacterial infections, diabetes, and fractures.”
“The long-term use of ICS is associated with several significant adverse events including pneumonia, mycobacterial infections, diabetes, and fractures,” reported Dr. Kenneth Chapman, Director of the Asthma and Airway Centre, University of Toronto Health Network, Toronto. One of several experts concerned about the overuse of ICS-containing combinations, Dr. Chapman presented a large, multinational trial at the 2018 ATS meeting that evaluated a strategy to de-escalate patients who had been taking triple therapy for at least six months but had no more than one moderate-to-severe exacerbation in the previous 12 months.
In this randomized double-blind trial, called SUNSET, 1053 patients were randomized without any intermediate step to remain on triple therapy or de-escalate to the LABA/LAMA combination of indacaterol/glycopyrronium. The primary endpoint was lung function by forced expiratory volume in 1 second (FEV1). Rate of exacerbations was a secondary endpoint.
“The direct change to indacaterol/glycopyrronium led to a small decrease in lung function at four weeks [with no further decline past that point] but with no significant difference in the rates of COPD exacerbations between treatments,” Dr. Chapman reported.
SUNSET: Endpoint and Safety Results
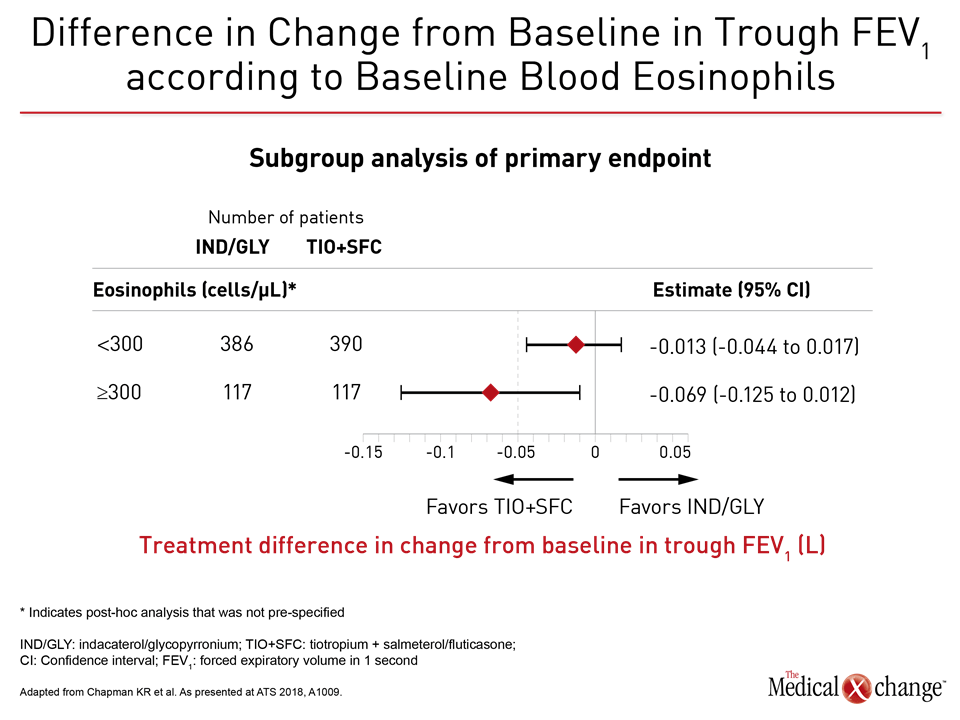
Due to the fact that the lower confidence interval of -0.053 of the difference in trough FEV1 (L) for the LABA/LAMA arm relative to ICS/LABA extended just beyond the pre-specified non-inferiority margin of -0.050 L, the study missed its non-inferiority endpoint. However, the median difference in trough FEV1 of -0.026 L was not only small overall but the difference was significant only for those with a baseline eosinophil count greater than 300 cells/μL (Fig. 2).
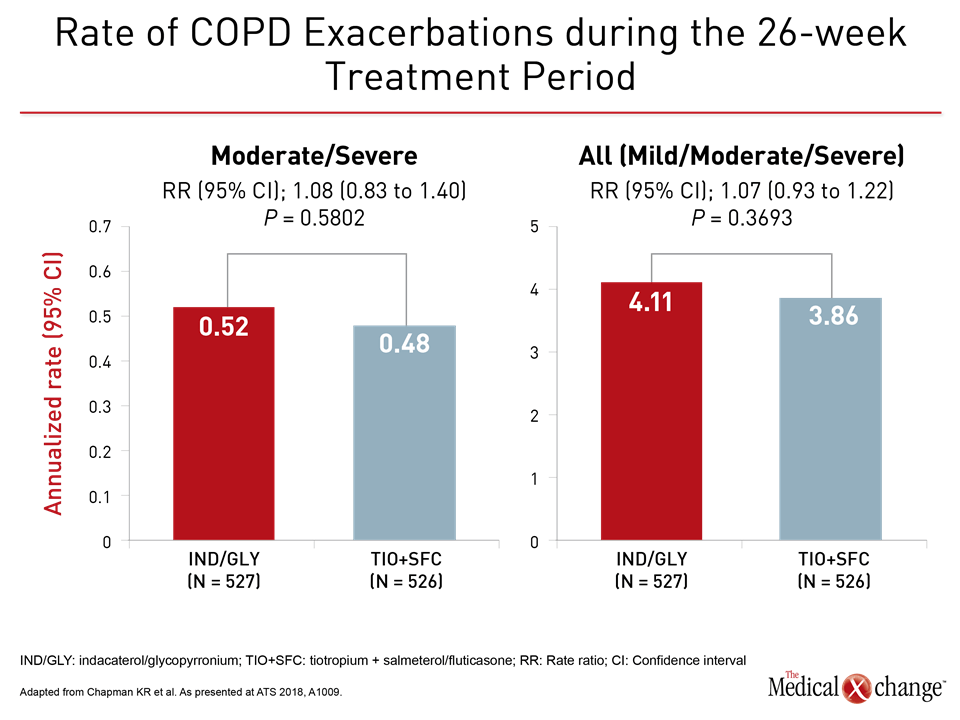
For the secondary but perhaps more clinically-relevant endpoint of exacerbations, there was no difference between arms in rate of moderate-to-severe exacerbations or in rate of all exacerbations (Fig. 3a and 3b).
“For the majority of these patients, the switch to indacaterol/glycopyrronium [from triple therapy] did not have any impact on lung function or exacerbations.”
“For the majority of these patients, the switch to indacaterol/glycopyrronium did not have any impact on lung function or exacerbations, while avoiding long-term exposure to ICS and related adverse events,” Dr. Chapman said. Study results raised no safety issues with regards to directly switching patients from triple therapy to a LABA/LAMA combination. Despite a modest decline in lung function after triple therapy de-escalation, Dr. Chapman indicated that the message from this study is that therapy should be individualized, not that patients without frequent exacerbations should remain on triple therapy.
Biomarkers for Triple Therapy Sought
“There was a difference in both exacerbations and lung function in patients with high blood eosinophils (≥300 cells/μL while on triple therapy) suggesting that it is these patients who will most likely benefit from continuation of triple therapy,” Dr. Chapman explained. As a result he believes this, another biomarker, or some combination of characteristics is likely to emerge for determining when patients without frequent exacerbations are candidates for triple therapy.
Even though the GOLD recommendations accept triple therapy as a reasonable step in patients with frequent exacerbations, there are very few head-to-head trials designed to compare this step to dual bronchodilators. To address this question, DACCORD, a real-world observational study, collected data in Germany from COPD patients treated with LABA/LAMA and those treated with triple therapy. Rate of exacerbations was compared over a one-year period.
This study matched data from more than 4000 patients on LABA/LAMA or triple therapy for age, gender, body mass index (BMI), smoking, health status, percent FEV1 predicted, and exacerbation history. After matching, 1046 pairs of patients on LABA/LAMA or triple therapy were followed for one year after either remaining on triple therapy or from a point of a baseline switch in maintenance therapy, such as from a single to a dual bronchodilator or from a dual bronchodilator to triple therapy.
Largest Benefit from Second Bronchodilator
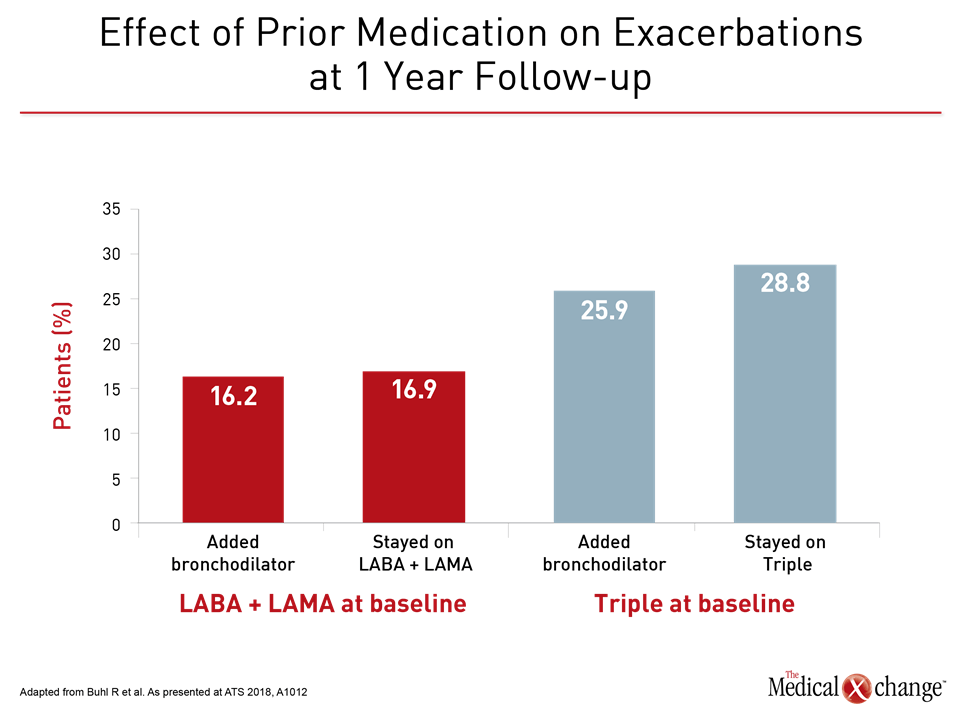
“The greatest benefit from the COPD maintenance therapy change on entry was in patients who added a second bronchodilator to a single bronchodilator. This benefit was greater than that observed in patients who added another bronchodilator to an ICS plus bronchodilator. Surprisingly, patients who stayed on triple therapy were at highest risk of exacerbating,” reported Dr. Roland Buhl, Johannes Gutenberg University, Mainz, Germany. By benefit, Dr. Buhl was referring to relative protection against COPD exacerbations (Fig. 4).
“The greatest benefit from the COPD maintenance therapy change on entry was in patients who added a second bronchodilator to a single bronchodilator.”
These data do not suggest that ICS increases the risk of an exacerbation, Dr. Buhl specified. Rather, he speculated that patients on ICS had more advanced disease despite the fact that they were matched to characteristics including baseline lung function. The fact that 38.4% of those in the triple therapy group versus <5% of those in the LABA/LAMA group had been taking triple therapy prior to enrollment also supports the theory that treating physicians believed this group required more therapy despite the similarity of the baseline characteristics. However, based on a lower rate of exacerbations in the dual bronchodilator group over the study period, the data indicate that these patients were being treated adequately, a message consistent with the current effort to induce closer adherence to current guidance documents.
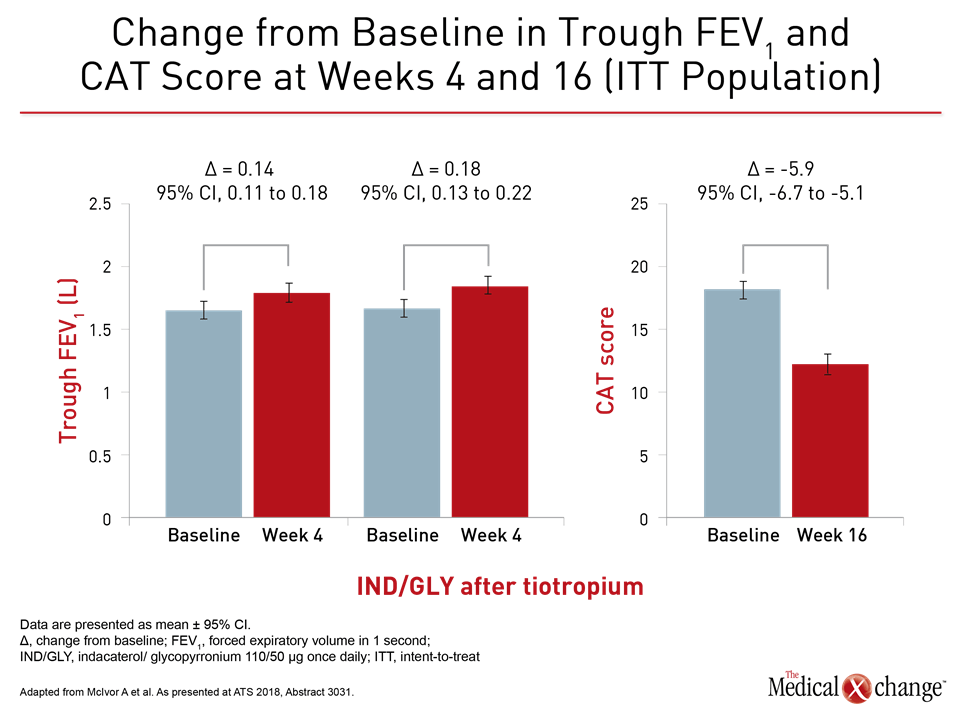
The same message was conveyed by results of the POWER study, which was also presented here. In this multicenter interventional study, 373 symptomatic moderate-to-severe COPD patients on the LAMA tiotropium or on dual LABA/ICS therapy of salmeterol/fluticasone at baseline were switched to once-daily treatment with indacaterol/glycopyrronium. There was no washout period. Changes in a variety of clinical assessments were measured at 16 weeks.
“Once-daily indacaterol/glycopyrronium demonstrated a statistically significant and clinically meaningful improvement in lung function, dyspnea, and health status,” reported Dr. Andrew McIvor, Firestone Institute for Respiratory Health, McMaster University, Hamilton, Ontario (Fig. 5). He suggested that the findings contribute evidence of the real-life effectiveness of switching directly to a LABA/LAMA in COPD patients with persistent symptoms on ICS/LABA or LAMA alone.
Conclusion
In Canada as elsewhere, ICS-based combinations are being inappropriately employed in patients without frequent exacerbations. This practice departs from current guidelines and is in conflict with current evidence that shows greater protection against exacerbations in COPD from a LABA/LAMA than ICS/LABA. The SUNSET trial showed no increased risk of exacerbations from moving patients without frequent exacerbations from triple therapy to dual bronchodilators with the potential exception of those who continued to have high eosinophil counts when on triple therapy. These data are consistent with guidelines that advocate bronchodilators over ICS-containing COPD regimens except in patients with frequent exacerbations.