Cardiology
Heart Failure 2018 & World Congress on Acute Heart Failure
Ensuring that All Heart Failure Patients Receive Optimal Treatment
Vienna – Unmet needs in heart failure (HF) were the focus of many presentations at this year’s Heart Failure Congress. A major limitation in improving the outcomes of patients with heart failure with reduced ejection fraction (HFrEF) is that therapies are not reaching all who could benefit– even though numerous well-conducted, randomized controlled trials have proven the benefit of a range of therapies in terms of morbidity, mortality, and improved quality of life (QOL), and despite having been incorporated into treatment guidelines. Another challenge is the lack of evidence-based therapies for HF with mid-range ejection fraction (HFmEF). In acute HF (AHF), despite multiple trials in the past few years, no evidence-based algorithms, treatments, or interventions have been shown to improve outcomes.
Incremental Progress in HFrEF Treatment
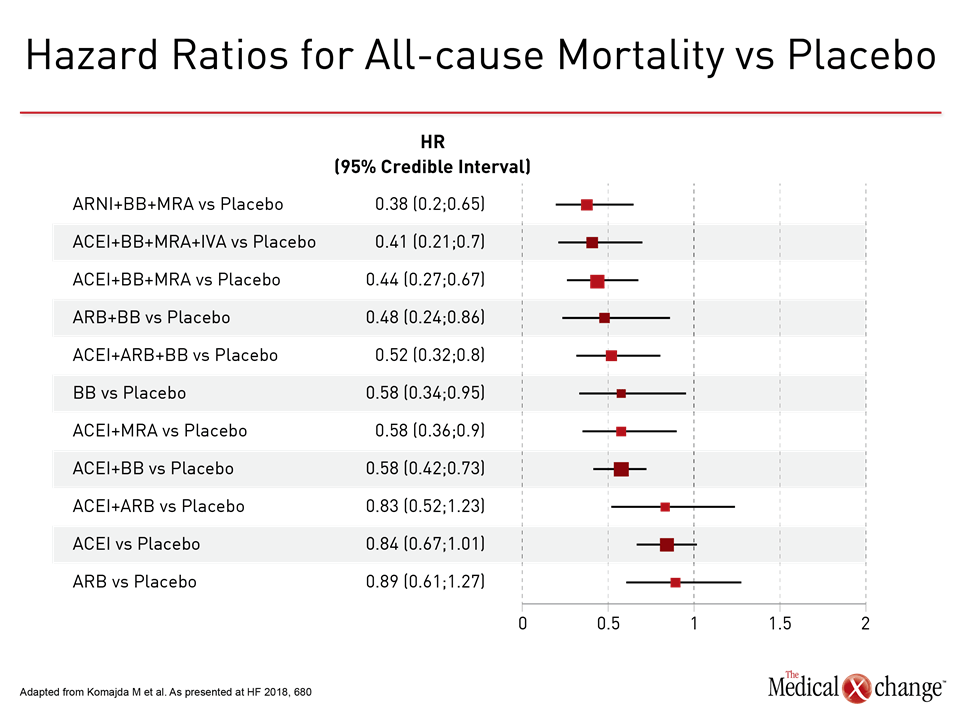
The progressive improvement made in the treatment of HFrEF with combination therapy over the past 30 years was demonstrated by the results of a network meta-analysis presented here by Dr. Michel Komajda, Department of Cardiology, Hôpital Saint Joseph, Paris, France (Komajda M et al. Eur J Heart Fail. May 27, epub ahead of print). The combinations that showed the greatest effects on major outcomes were those made up of an angiotensin receptor-neprilysin inhibitor (ARNI; sacubitril/valsartan), a beta-blocker (BB) and a mineralocorticoid receptor antagonist (MRA), or an angiotensin-converting enzyme inhibitor (ACEI), BB, MRA, and ivabradine (IVA), a sinus node If channel inhibitor.
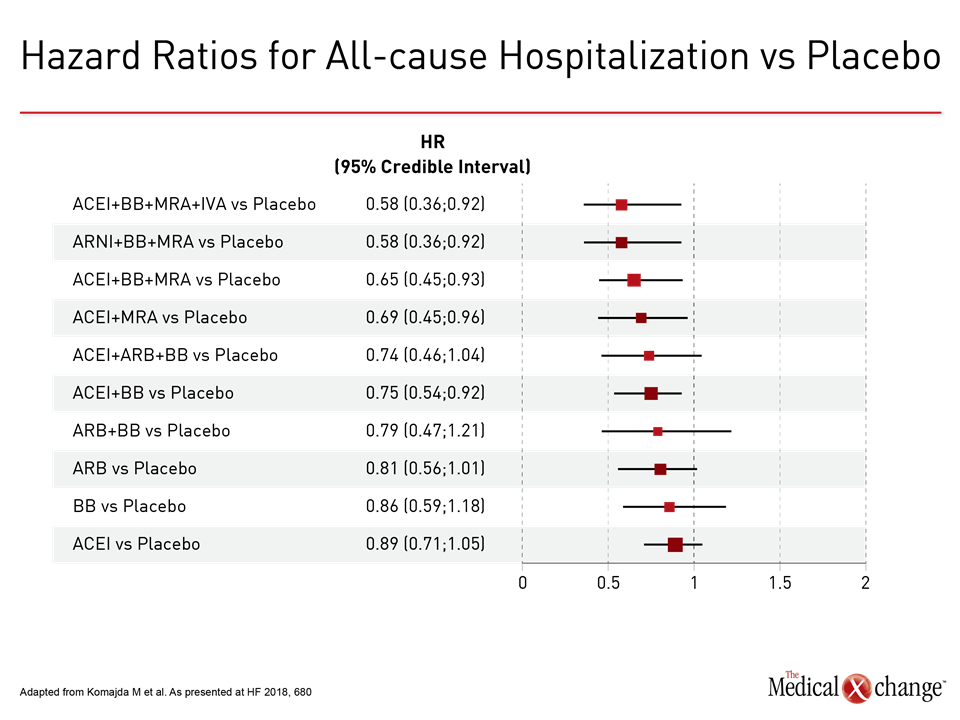
Dr. Komajda and his colleagues reviewed data from 58 clinical trials of treatment guideline-recommended drug classes in chronic HFrEF (EF <45%) published between 1987 and 2017. Compared with placebo, the ARNI+BB+MRA and ACEI+BB+MRA+IVA combinations were associated with the biggest risk reductions in all-cause mortality (62% and 59%, respectively) (Fig. 1)and in cardiovascular (CV) mortality (64% and 59%). A 42% risk reduction in all-cause hospitalization was seen with both combinations (Fig. 2)and 73% and 75%, respectively, in hospitalizations due to worsening HF. These data support current international guideline recommendations for management of HFrEF, Dr. Komajda noted.
Congress discussant Prof. Mitja Lainscak, General Hospital Murska Sobota and Faculty of Medicine, University of Ljubljana, Slovenia, urged wider use of these combination therapies, adding that “we don’t implement the guidelines as we should.”
Extrapolating from Guideline Recommendations
In 2016, major new HF guidelines or updates were issued by the ESC (Ponikowski P et al. Eur Heart J. 2016;37:2129-200) and the American College of Cardiology (ACC)/American Heart Association (AHA) (Yancy C et al. J Am Coll Cardiol. 2016;68:1476-88), followed in 2017 by the Canadian Cardiovascular Society (CCS) (Ezekowitz JA et al. Can J Cardiol. 2017;33:1342−433). All the guidelines added recommendations for an ARNI (sacubitril/valsartan) to be administered in place of an ACEI or ARB in patients who remain symptomatic despite guideline-directed medical therapy.
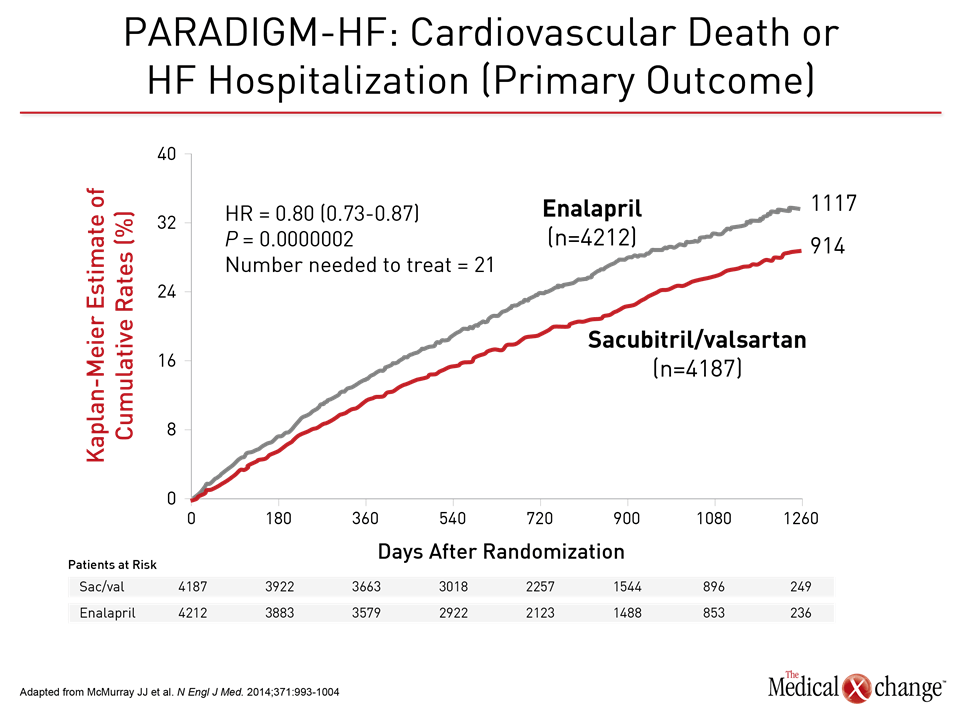
This recommendation was based on the results of the PARADIGM-HF trial, in which ARNI added to standard care was associated with a 20% decrease in the composite primary endpoint of death from CV causes or hospitalization for HF, as well as reductions of 20% in cardiovascular death (CVD) (Fig. 3), 21% in first hospitalization for worsening HF, and 16% in all-cause mortality, and improvements in quality of life (McMurray JJ et al. N Engl J Med. 2014;371:993-1004).
Prof. Andrew Coats, Monash University, Australia, and University of Warwick, UK, suggested that the PARADIGM-HF results could be extrapolated to patients who did not fulfill all the entry criteria of the trial, particularly those related to age, BNP level, or blood pressure. He suggested that “it might be possible to start ACEI-naïve patients on half-dose ACEI before switching to sacubitril/valsartan,” provided that it was done in a way that was similar to the trial.
Dr. Milton Packer, Baylor University Medical Center, Dallas, Texas, US, noted that since an ARNI is now recommended by the guidelines, usual care expands their use beyond the criteria of a trial in general, including PARADIGM-HF. This treatment option is becoming more common in clinical practice as healthcare professionals become more familiar with this therapy. If a patient “violates” one of the PARADIGM-HF entry criteria, it should not prevent them from being prescribed sacubitril/valsartan, even without previous ACEI therapy, he urged.
Real-world Experiences vs Clinical Trials
Real-world experiences with new therapies may not mirror the clinical trial experience, as demonstrated by observational studies carried out at institutions where stricter eligibility criteria are applied. For instance, unlike the entry criteria for PARADIGM-HF, the 2016 ESC HF guidelines specify that patients eligible for an ARNI must be symptomatic (NYHA II and III) after optimal therapy that includes an MRA. Researchers from Our Lady of Lourdes Hospital, Drogheda, Ireland, which adheres to the ESC guidelines, believe that this extra step in the treatment algorithm may prevent patients who could benefit from sacubitril/valsartan from receiving it. Dr. Tim O’Connor reported that of 434 patients referred with HFrEF over a 2-year period, only 27/116 (23%) of those with EF ≤35% met ESC criteria for an ARNI. The main reason for ineligibility was an insufficient dose of ACEI/ARB (58%), followed by being asymptomatic and having low SBP <95 mmHg or GFR <30 mL/min. Dr. O’Connor called for more flexibility in the eligibility criteria to allow more patients to receive sacubitril/valsartan.
The Myth of Clinical Stability in “Mild” HF
Two reasons why patients are not receiving optimal treatment are that physicians believe in patients with “mild HF” who are “stable on their current therapy,” which leads to a false sense of security, declared Dr. John McMurray, BHF Cardiovascular Research Centre, University of Glasgow & Queen Elizabeth University Hospital, Scotland. “There is no such thing as ‘mild HF,’” he declared. “There are HF patients with mild symptoms, but those patients progress rapidly, even on optimal treatment,” he said. “The idea that any patient with HF is stable, including those with mild symptoms, well treated, and no recent hospital admissions, is a myth.”
“The idea that any patient with HF is stable, including those with mild symptoms, well treated, and no recent hospital admissions, is a myth.”
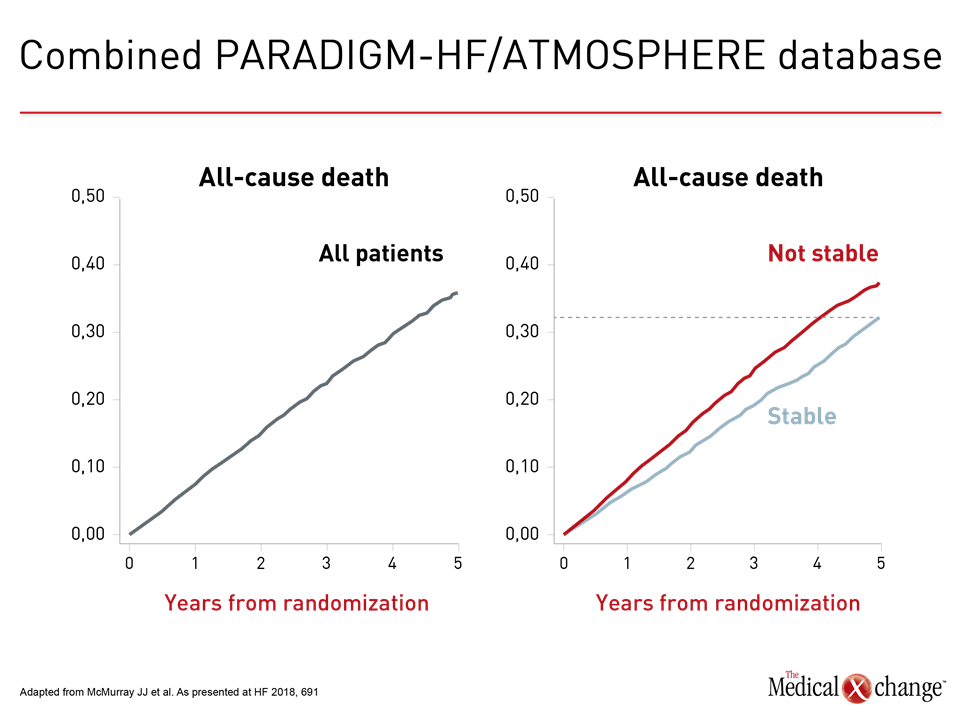
This was demonstrated by an analysis of pooled data from the 14,415 patients with HFrEF in the PARADIGM-HF and ATMOSPHERE (McMurray JJ et al. N Engl J Med. 2016;374:1521-32) trials. These patients had a mean age of 63 years and were well treated by conventional standards. Among patients in NYHA Class II, with mild symptoms, who had not been hospitalized with HF in past 12 months, i.e., “stable,” one third were dead within 5 years, Dr. McMurray observed (Fig. 4). Around 40% had either died of a CV cause or been hospitalized at least once with HF. Even within one year, about 10% had experienced one major adverse CV event. By 4 months, 25% of patients had a 5-point decrease in the Kansas City Cardiomyopathy Questionnaire (KCCQ), considered a clinically important deterioration in quality of life (QOL) and associated with worse clinical outcomes.
“We need to do more for our patients. If we suffer clinical inertia, which is a normal human tendency, our patients will not do as well as they could,” Dr. McMurray declared. “The sooner you act the better, because all these treatments work very quickly,” he urged, noting that in PARADIGM-HF, there was a significant (35%) improvement in outcomes as early as 30 days after randomization.
Heart Failure with Mid-range Ejection Fraction
A recent development in HF guidelines was the introduction of heart failure with mid-range EF (HFmEF), defined in the 2017 CCS guideline as EF 41-49%. One reason for the introduction, Prof. Coats explained, was to encourage more clinical trials and analyses of this group. “We did not know whether pathophysiologically HFmEF was like HFrEF or heart failure with preserved EF (HFpEF),” he admitted.
“We did not know whether pathophysiologically HFmEF was like HFrEF or heart failure with preserved EF (HFpEF).”
For treatment to date, “I believe that for HFmEF, candesartan and BBs are established clinically, not at a level of guidelines’ strict criteria, but at a common sense level, based on what the available evidence tells us,” Prof. Coats said.
The benefit of candesartan was shown in an analysis of 1322 patients with HFmEF who participated in the CHARM program and showed a statistically significant reduction (24%) in the primary outcome of CVD or HF hospitalization (Lund LH et al. Eur J Heart Fail. February 12, epub ahead of print). This was not a prespecified subgroup, so it is not proof, but it is another level of evidence, Prof. Coats commented.
In a meta-analysis of data from 11 BB trials, statistically significant reductions of 41% in all-cause mortality and 52% in CV mortality were demonstrated in the 575 patients with EF 40-49% (Cleland JGF et al. Eur Heart J. 2018;39:26-35).
Prof. Coats suggested these analyses be included in the next HF guidelines, although they would not be given the same strength of recommendation as a prospectively designed clinical trial.
Drug Therapy for Acute Heart Failure
According to major guidelines, the mainstay of treatment for AHF remains the administration of diuretics as first-line therapy, despite multiple studies showing that higher doses of diuretics are associated with poorer outcomes, and despite the development of resistance, noted Prof. Marco Metra, Institute of Cardiology, University of Brescia, Italy.
In recent years, clinical trials with other therapies, including different modes of administration of furosemide and so-called “renal protective” drugs (rolofylline, low-dose dopamine, nesiritide) showed no effects on clinical outcomes.
Early studies of torsemide suggested that it may be an alternative to furosemide, with potentially better outcomes in AHF (Bikdeli B et al. J Am Coll Cardiol. 2013;61:1549-50). A US randomized clinical trial, TRANSFORM-HF is comparing the effects of the 2 agents on all-cause mortality in 6000 patients (Fig. 5). Trials of addition of other diuretics or alternative drugs to diuretics have been mainly neutral, Prof. Metra observed.
The hypothesis that short-term treatment could affect long-term outcomes has been tested in two clinical trials, with ularitide (TRUE-AHF) and serelaxin (RELAX-AHF-2), but both failed, Prof. Metra noted. “Long-term treatment in AHF must be optimized during hospitalization before discharge,” he stressed.
An option in patients hospitalized for acute decompensated HF (ADHF) may be sacubitril/valsartan, according to a cohort study presented by Dr. Luiz CS Passos, Federal University of Bahia, Salvador, Brazil. Among 543 patients with EF<50%, 198 (36.5%) were found to be eligible for treatment according to PARADIGM-HF selection criteria.
Conclusion
Effective treatments for HFrEF are widely available, but greater efforts are needed globally to ensure that they reach the patients who could benefit from them. This could be achieved by initiatives to improve prescription of guideline-recommended therapies, patient education and engagement, and post-discharge planning for patients with HF. The complexity of all the evidence-based treatment options in HF presents new challenges. Further investigation is needed into the pathophysiology of HFmEF and AHF to allow development of effective therapies.