rheumatology
European League Against Rheumatism (EULAR) 2018
JAK Inhibitors Test Specificity of Action in Rheumatoid Arthritis
Amsterdam – New Janus kinase (JAK) inhibitors are testing the premise that affinity for the JAK1 pathway is a determinant of clinical benefit in rheumatoid arthritis (RA). Neither tofacitinib, the first approved JAK inhibitor, nor baricitinib, which was recently granted approval for RA in the US, offer a high degree of relative selectivity on the JAK1 pathway. In contrast, both of the newer agents in ongoing clinical studies, upadacitinib and filgotinib, show a far greater affinity for JAK1 relative to the other isoforms. At the 2018 EULAR Congress, high rates of activity in RA patients poorly responsive to conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) were associated with upadacitinib in phase 3 trials and filgotinib in a phase 2 trial. Although direct comparisons of the JAK inhibitors have yet to be conducted, the precision of JAK targeting has the potential to fundamentally alter the benefit-to-risk ratio of agents in this class.
JAK Signaling: Finding the Therapeutic Window
The activity of tofacitinib has demonstrated that inhibition of JAK-STAT signaling is effective in the treatment of RA and other inflammatory diseases, but it is a relatively non-selective JAK inhibitor, leaving open the possibility of greater relative effects from greater selectivity. The four JAK proteins, JAK1, JAK2, JAK3 and TYK2, govern a variety of cellular functions of which only a few would be expected to offer clinical benefit. The putative target in RA is JAK1, which regulates expression of IL-6 and interferon alpha. Conversely, inhibition of other JAK pathways, such as JAK2, which regulates erythropoiesis, or JAK3, which is associated with NK cell regulation, creates a substantial potential for adverse impact on the risk-to-benefit ratio of any given agent.
“The aim in the development of the two JAK1-selective drugs has been to identify the therapeutic dose that targets JAK1 without too much effect on the other JAKs. In other words, a therapeutic window,” explained Dr. John D. Isaacs, Institute of Cellular Medicine, University of Newcastle, UK. No JAK inhibitor that has reached clinical development offers pure inhibitory effects on any of the four JAK pathways. Rather, the relative effects on each introduce very different potential ratios of benefit to risk.
Clinical data are essential because the therapeutic profile is not just determined by activity on one JAK pathway or lack of activity on another, according to Dr. Isaacs. Rather, other factors unique to each JAK pathway inhibitor are important.
Pharmacokinetics Influence Drug Activity
“It is not only the relative potency towards the different JAK enzymes, which is what is called selectivity, but the intracellular concentration, because the ability of the drug to get into the cell depends on pharmacokinetics. Concentration is important for inhibiting the target JAK enzyme, but there can be loss of selectivity if a JAK inhibitor enters the cell at very high concentrations,” Dr. Isaacs explained.
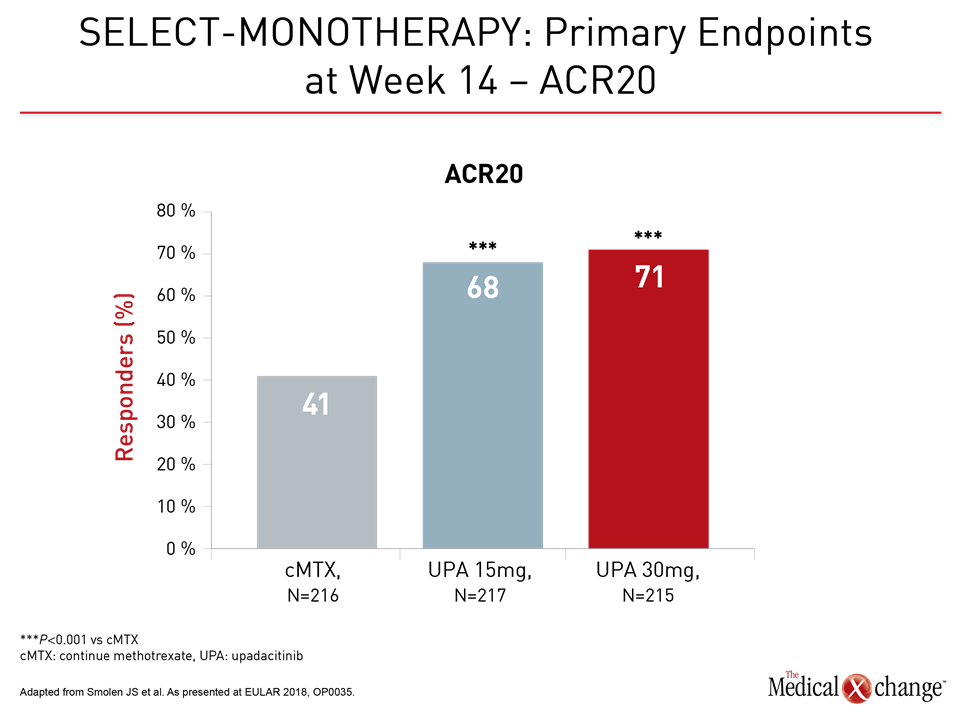
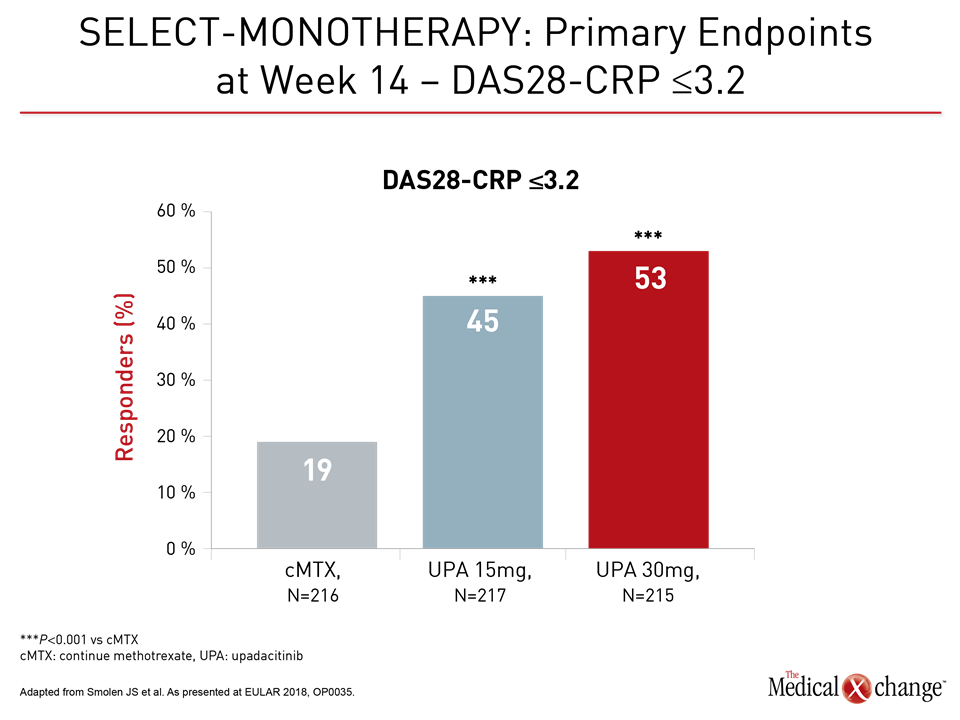
Several sets of phase 3 data with upadacitinib were presented at this year’s EULAR Congress. In the SELECT-MONOTHERAPY trial, 648 patients were enrolled. All had an inadequate response to at least one csDMARD and continued to experience moderate-to-severe RA despite methotrexate (MTX) therapy. They were randomized to 15 mg of upadacitinib once daily, 30 mg of upadacitinib once daily, or to remain on methotrexate. The primary endpoints were proportion of patients achieving reduced disease activity as defined by ACR20 or DAS28-CRP ≤3.2 (Fig. 1a and 1b).
The treatment benefit of upadacitinib relative to methotrexate was large and highly significant by either set of criteria. In addition, there was a significant advantage for upadacitinib relative to methotrexate for all key secondary endpoints, including ACR50, ACR70, Health Assessment Questionnaire without Disability Index (HAQ-DI) and morning stiffness duration.
Remission Rate Approaches 20% at 14 Weeks
Compared to 1% in the methotrexate group, clinical remission at 14 weeks, defined as a Simplified Disease Activity Index (SDAI) score ≤3.3, was achieved in 14% of those randomized to 15 mg upadacitinib and 18% of those randomized to 30 mg upadacitinib, reported Dr. Josef Smolen, Department of Rheumatology, University of Vienna, Austria. By the DAS28-CRP definition of <2.6, these remission rates were 8%, 28%, and 41%, respectively.
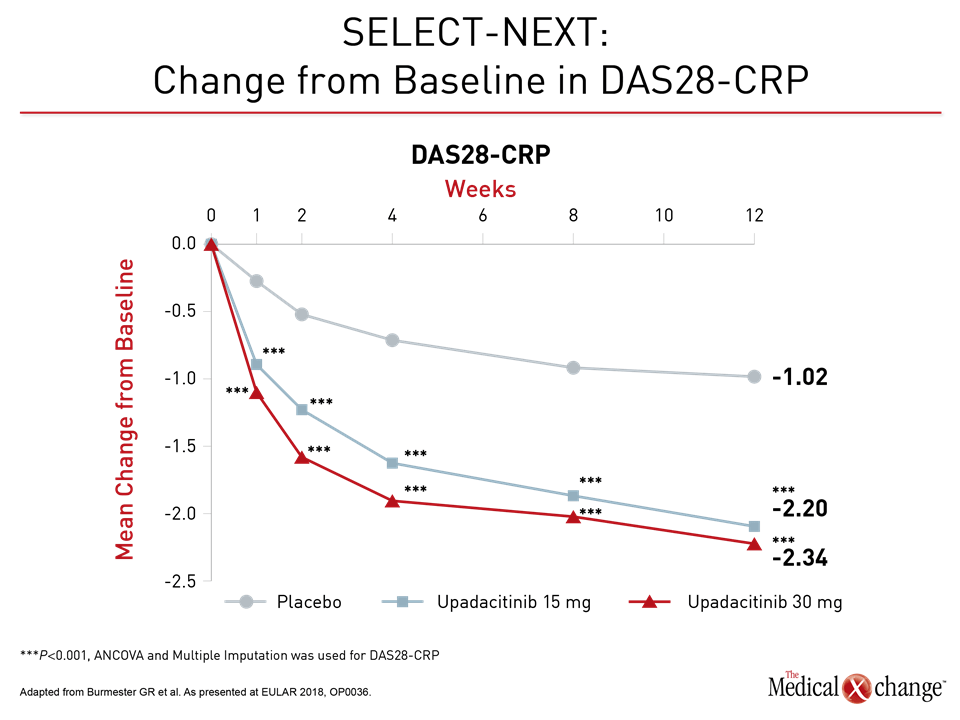
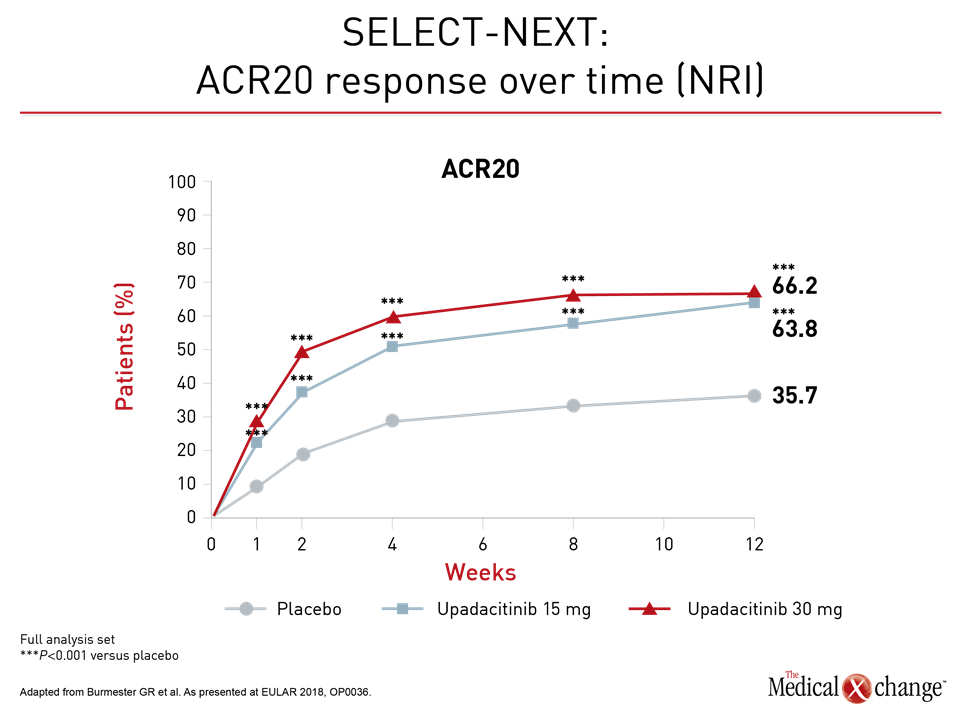
Similarly high rates of response and remission were also achieved quickly in the phase 3 trial, called SELECT-NEXT. The 661 patients in this study also had a history of an inadequate response to csDMARDs and were randomized to 15 mg upadacitinib, 30 mg upadacitinib, or placebo. Patients in this trial were allowed to remain on their background csDMARDs. In the data presented at the 2018 EULAR Congress, the rates of ACR20 response at week 12 for 15 mg upadacitinib (64%) and 30 mg upadacitinib (66%) were almost double that of placebo (36%; P<0.001 versus either upadacitinib dose).
Furthermore, responses were rapid, according to Dr. Gerd R. Burmester, Department of Rheumatology and Clinical Immunology, Charité University Clinic, Berlin, Germany. He showed graphs of meaningful and statistically significant disease control within one week of treatment initiation (Fig. 2a and 2b).
Again, as in SELECT-MONOTHERAPY, the rapid onset of activity led to very high rates of disease control and remission by the end of study period in SELECT-NEXT. For remission as defined by SDAI (≤3.3) the rate at 12 weeks climbed from 3% in the placebo group to 10% (P<0.01) in the 15 mg upadacitinib arm and 12% (P<0.001) in the 30 mg upadacitinib arm, or three to four times higher. For DAS28-CRP (<2.6), the rates at 12 weeks were 10%, 31%, and 28% (both P<0.001 vs. placebo), respectively, Dr. Burmester reported.
Safety Supported by Growing Data Pool
Adverse events in this study and another phase 3 trial updated here, SELECT-BEYOND, were consistent with the phase 2 experience. Serious adverse events overall and those leading to discontinuation have not been consistently higher with either dose of upadacitinib when compared to placebo. In SELECT-NEXT, for example, the rate of serious adverse events was 2.3% in the placebo group and 2.7% in the 30 mg upadacitinib arm. The rate of serious adverse events was 4.1% in the 15 mg upadacitinib arm, but the rate of discontinuations due to adverse events was 3.2%, which was the same as that in the placebo arm.
Speed of Response to Therapy
In the update of SELECT-BEYOND data, which randomized 499 patients with an inadequate biologic DMARD (bDMARD) response to upadacitinib or placebo, the speed of response after initiating upadacitinib again drew the attention of lead author, Dr. Mark C. Genovese, Division of Immunology/Rheumatology, Stanford University, California.
“By the first week, the proportion of patients who achieved ACR20 was more than double on 15 mg upadacitinib (27.4%) or 30 mg upadacitinib (24.8%) when compared to placebo (10.7%; P<0.001),” Dr. Genovese reported. He indicated that is a particularly meaningful advantage for those with persistent symptoms on csDMARDs or bDMARDs.
By the first week, the proportion of patients who achieved ACR20 was more than double on 15 mg or 30 mg upadacitinib when compared to placebo.
Sustained Long-Term Response
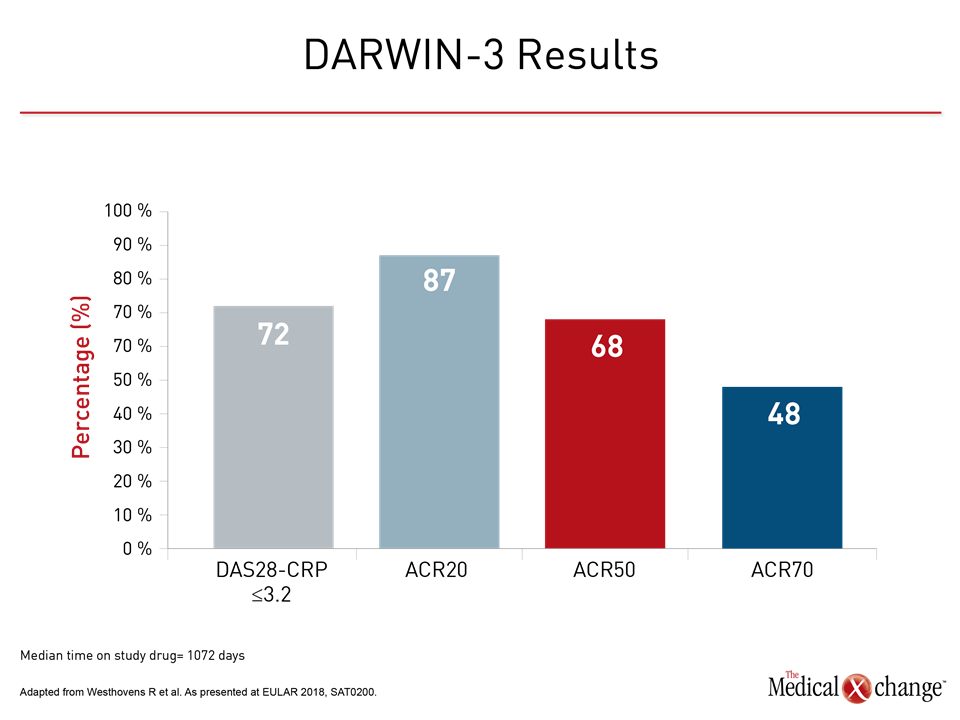
The trials with filgotinib are at an earlier stage, but a phase 2b open-label extension study of the DARWIN clinical trials program also supports on-going development of this JAK inhibitor. Safety was the primary objective of this extension trial, called DARWIN-3, presented at the 2018 EULAR Congress, but the sustained activity was impressive. With a median time on drug of 1072 days, the rates of response at last follow-up, whether measured by ACR20, ACR50, ACR70, or DAS28-CRP ≤3.2, were sustained, according to Dr. Rene Westhovens, Professor of Rheumatology, University of Leuven, Belgium (Fig. 3).
“We can also say that filgotinib continues to be well tolerated over prolonged exposure,” reported Dr. Westhovens, who identified no new safety signals in 1,931 patient-years of exposure. In his remarks, he also noted a rapid onset of action with filgotinib, a potential advantage that is potentially linked to its relatively strong JAK1 affinity.
JAK1 selectivity for tofacitinib assessed through enzyme assays, expressed as 50% in vitro inhibitory concentration (IC50), is 15.1 nM, but relatively low concentrations are also required for inhibition of both JAK2 (77.4 nM) and JAK3 (55 nM). Similarly, the IC50 for baricitinib is 4.0, but the IC50 for JAK2 is only 6.6 nM. This confers a low relative level of selectivity for the JAK1 and JAK2 pathways. In contrast, although the IC50 of upadacitinib for JAK1 is 47 nM, it rises to 120 nM for JAK2 and to 2304 nM for JAK3. Filgotinib requires the highest IC50 for JAK1, which is 363 nM, but the IC50 for JAK2 is 2400 nM and exceeds 10,000 NM for JAK3, providing this agent, like upadacitinib, with relative JAK1 selectivity at any given dose.
Selectivity in a Clinical Context
The relevance of these selectivities for clinical benefits or clinical risks is unknown. Moreover, Dr. Isaacs, who reviewed these data in a symposium at the EULAR 2018 Congress, cautioned that in vitro selectivity cannot be evaluated in isolation. Although correlations have been observed in the experimental setting between JAK selectivity and expected clinical activity, such as JAK1 inhibition and inhibition of cytokine IL-6, they are imperfect.
“These agents have drug exposure dependent effects that determine their relative selectivity for the four different JAK isoforms, but the clinical relevance of selectivity differences between currently available JAK inhibitors will be determined by clinical experience,” Dr. Isaacs advised. He called the assays “educational,” but cautioned that there “have been some paradoxes in the data,” necessitating clinical experience to confirm their relevance.
“There is some evidence that selective inhibition of JAK1 could lead to optimizing efficacy without significantly impacting parameters mediated by other JAK isoforms,” Dr. Isaacs said.
In addition, it is unclear what aspects of JAK selectivity will be important to clinical effects. In reviewing the clinical experience with each of the four JAK inhibitors available or in clinical trials, Dr. Andrea Rubbert-Roth, Senior Physician in the Clinic for Internal Medicine, University of Cologne, Germany, suggested that the evidence for major differences in efficacy and safety is limited in the absence of comparative studies. However, she, too, commented on the speed of onset of the more selective agents.
“Very impressively, and I can confirm this from my own experience as I have participated in the clinical trials, there is a significant response already after one week.”
“Very impressively, and I can confirm this from my own experience as I have participated in the clinical trials, there is a significant response already after one week,” said Dr. Rubbert-Roth, referring specifically to upadacitinib. “This is dramatic. If you see patients after one week, you do not really expect a lot of efficacy at that point. When I hear patients say the swelling is better, the pain is better after such a short time, this is very encouraging.”
Conclusion
The concept of JAK inhibitor selectivity is becoming relevant as newer JAK inhibitors with concentrated effects on JAK1 reach routine clinical application. So far, the phase 3 data with upadacitinib and phase 2 data with filgotinib suggest a level of efficacy and safety at least commensurate with that of tofacitinib, the first agent in this class. Although Dr. Rubbert-Roth called JAK inhibitors “oral therapies with a biologic effect,” the potential for a more favorable therapeutic window with newer, more selective JAK inhibitors could move these therapies even further forward in the treatment algorithm.