Cardiology
American College of Cardiology (ACC) 2019 Scientific Sessions
Earlier Treatment Intervention in Heart Failure Patients Results in Improved Outcomes
New Orleans – For patients with acute decompensated heart failure (HF), new data confirm that the initiation of treatment prior to hospital discharge confers protection in the early post-discharge period. According to a more detailed analysis of the landmark PIONEER-HF trial, which was first presented in November 2018, in-hospital initiation of therapy advances the time to protection from HF events, covering the early period of vulnerability. While the published PIONEER-HF results demonstrated that in-hospital administration of a single-pill combination is well-tolerated and rapidly reduces the cardiac stress biomarker NT-proBNP, the new analyses correlate the decline in NT-proBNP with early and significant reductions in HF rehospitalization, implantation of a left ventricular assist device (LVAD), and death.
The single-pill combination of the neprilysin inhibitor sacubitril and the angiotensin receptor blocker (ARB) valsartan (sacubitril/valsartan) became a guideline-recommended therapy for heart failure (HF) patients with reduced ejection fraction (HFrEF) after results of the PARADIGM-HF trial were published almost five years ago. The trial demonstrated that sacubitril/valsartan is well-tolerated and reduces risk of death and hospitalization for HF relative to the angiotensin-converting enzyme inhibitor (ACEi) enalapril in a HFrEF population. The newly published PIONEER-HF trial (Velazquez EJ et al. N Engl J Med 2019;380:539-548) demonstrated that in-hospital initiation of sacubitril/valsartan is well-tolerated and reduces NT-proBNP concentrations. New PIONEER-HF substudies at this year’s ACC meeting establish clinical benefits.
Early Optimization of Therapy
“Given the substantial risk of readmission in the first 30 days after an index admission, the in-hospital initiation of sacubitril/valsartan represents an important opportunity to improve post-discharge HF outcomes,” reported Dr. Adam DeVore, Assistant Professor, Duke University Medical Center, Durham, NC. Dr. DeVore presented one of the new substudies from PIONEER-HF, in a special Featured Clinical Research session at the ACC.
In the newly-completed secondary analysis of PIONEER-HF, outcomes were evaluated in the 832 (94% of the 881 initially randomized) patients who participated in an open-label extension. At the start of the four-week open-label extension, which followed the eight-week randomized trial, patients in the enalapril group were switched to sacubitril/valsartan. Events over the 12 weeks were compared in the two groups.
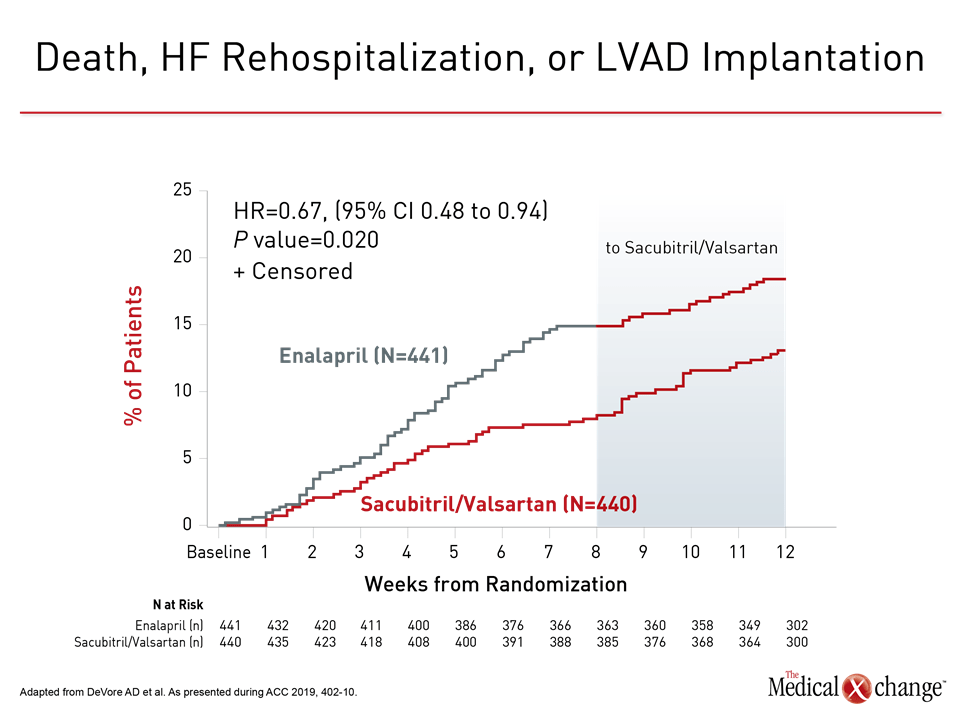
When evaluated at the end of 12 weeks, the risk of the composite endpoint of death, HF hospitalization, and LVAD implantation, was reduced by 33% (HR 0.67; P=0.02) for those initially randomized to sacubitril/valsartan relative to enalapril (Figure 1). When displayed graphically, the advantage of sacubitril/valsartan was observed within weeks of randomization and increased throughout the period of randomization. Although there was no further separation of the curves after enalapril patients were switched to sacubitril/valsartan, the advantage of starting on sacubitril/valsartan persisted until the end of the open-label extension.
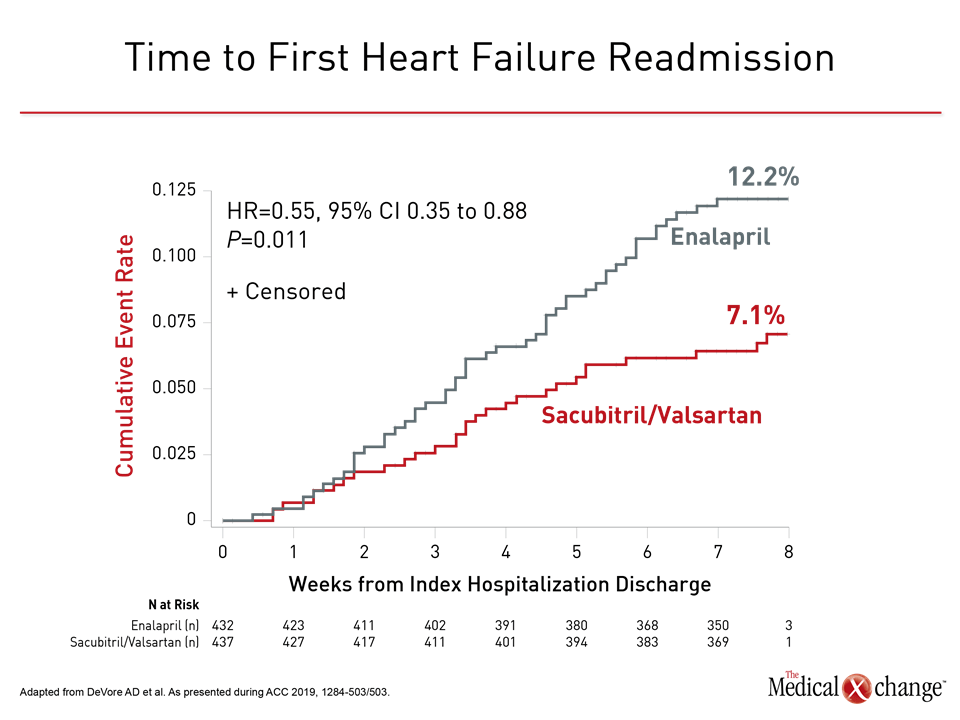
The value of early optimization of therapy for HFrEF was highlighted by the relative protection of sacubitril/valsartan against HF readmissions. When calculated for time to heart failure readmission, the curves for the study arms had diverged within three weeks. By eight weeks, 12.2% of those randomized to enalapril had an HF-related readmission versus only 7.1% of those randomized to sacubitril/valsartan. This represented a 45% relative reduction in risk (HR 0.55; P=0.011), and the differences in event rates were again apparent within weeks (Figure 2).
“We do know that the real vulnerable period for rehospitalization is early on. That’s when we need to have these patients on the best therapy.”
“We do know that the real vulnerable period for rehospitalization is early on,” Dr. DeVore said. “That’s when we need to have these patients on the best therapy.”
In the previously reported results from PIONEER-HF, which enrolled patients who had been admitted for acute decompensated HF, had a left ventricular ejection fraction (LVEF) of ≤40%, and an NT-proBNP concentration of ≥1600 pg/mL, patients were randomized to the experimental or control arms after they met all four criteria for achieving hemodynamic stability (Table 1). When the randomized groups were compared over the subsequent eight weeks of the trial, there were no significant differences in any serious adverse events, including symptomatic hypotension, hyperkalemia, or worsening renal function.
According to Dr. DeVore, these same criteria are appropriate in clinical practice. These are easily evaluated and were shown in PIONEER-HF to define a population that can tolerate and benefit from starting sacubitril/valsartan in hospital.
“The definition of hemodynamic stability was fairly straightforward,” said Dr. DeVore, who does not believe that screening for hemodynamic stability prior to initiating sacubitril/valsartan therapy is likely to either complicate in-hospital management or extend hospital stay. Although effect on hospital stay was not explicitly evaluated in PIONEER-HF, Dr. DeVore reported that the median stay was five days. Referring to data for hospitalizations for acute decompensated HF in the United States, he said this was “similar to the national average.”
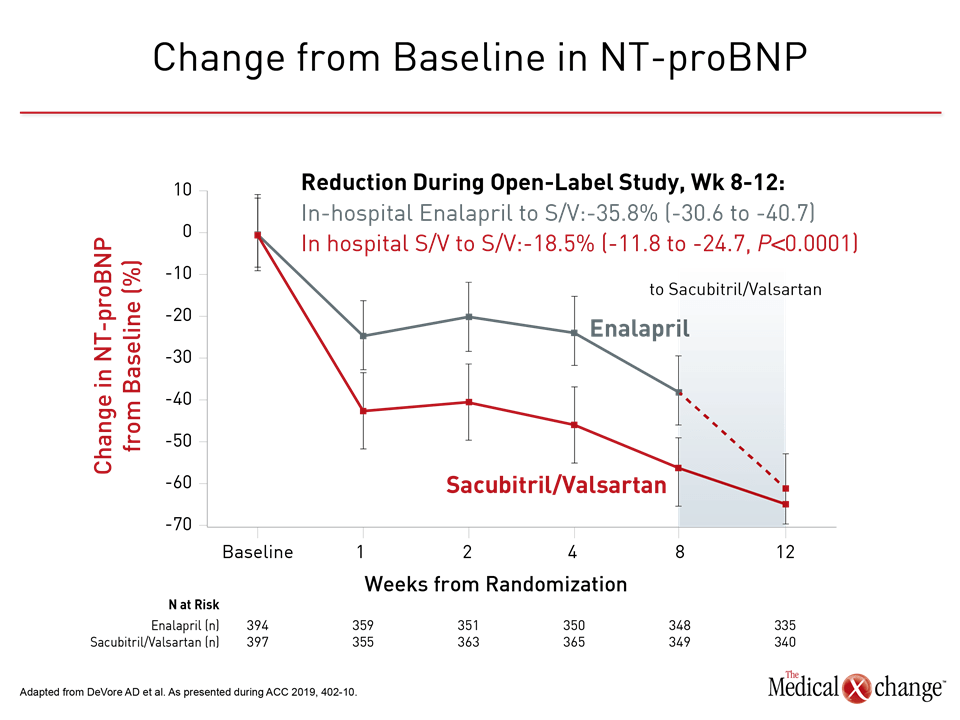
Supporting the underlying hypothesis of PIONEER-HF, the improvement in outcomes in patients taking sacubitril/valsartan was inversely related to levels of NT-proBNP. These levels fell faster on sacubitril/valsartan relative to enalapril and remained consistently lower throughout the period of randomization. At eight weeks, after those initiated on enalapril were switched to sacubitril/valsartan, NT-pro-BNP levels continued to decline in both groups of patients. The reduction in NT-proBNP in those initiated on enalapril did not catch up to those initially randomized to sacubitril/valsartan until the end of the open-label extension (Figure 3).
Additional Protection Against HF-related Events
The value of sacubitril/valsartan for providing incremental additional protection against HF-related events is attributed at least in part to neprilysin inhibition. Neprilysin is an important and well-established mediator of neurohormonal activation, that drives cardiac remodeling and ultimately clinical events in patients with HF. When combined with the angiotensin system inhibition provided by valsartan, sacubitril has added effects on markers of cardiac stress.
This effect was evaluated in another prespecified analysis from PIONEER-HF in which change in high-sensitivity troponin (hsTnT), which is a measure of muscle injury, soluble ST2 (sST2), which is a measure of ventricular wall stress, and urinary cyclic GMP (ucGMP), which reflects activation of natriuretic peptide receptors were compared in the sacubitril/valsartan and enalapril groups.
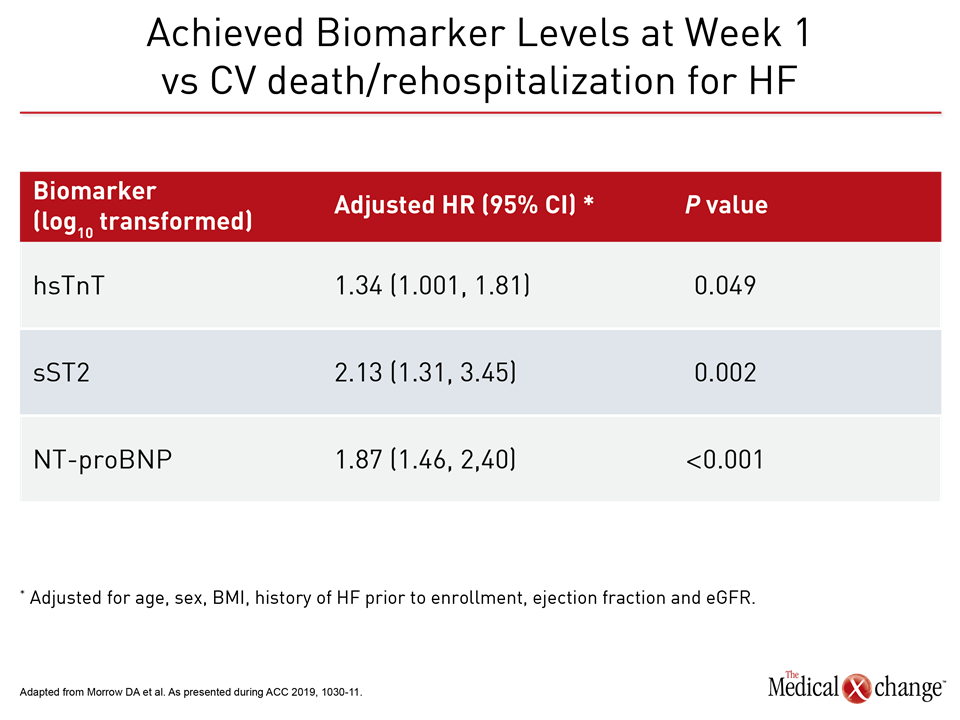
“The achieved biomarker concentration at week one was associated with subsequent CV death or rehospitalization for heart failure through week eight.”
A greater decline occurred more quickly on sacubitril/valsartan relative to enalapril for all three cardiac stress biomarkers, according to the first author of this subanalysis, Dr. David A. Morrow, Director of the Cardiac Intensive Care Unit, Brigham and Women’s Hospital, Boston, MA. Furthermore, data from PIONEER-HF suggested early declines in these markers of cardiac stress are prognostically important.
“The achieved biomarker concentration at week one was associated with subsequent cardiovascular (CV) death or rehospitalization for HF through week eight,” Dr. Morrow reported (Table 2).
Earlier Intervention in Patients with HFrEF
All of the recent findings advance an important narrative in the understanding of HF that began when the PARADIGM-HF trial associated sacubitril/valsartan with a 20% reduction (P<0.001) in CV death and HF rehospitalizations relative to enalapril after a median of 27 months of follow up (McMurray JJV et al. N Engl J Med 2014;371:993-1004). In that trial when there was relatively limited experience with sacubitril/valsartan, safety was an important focus. The design included a long run-in phase and then a slow upwards titration. PIONEER-HF enrolled a higher proportion of patients in New York Heart Association (NYHA) class III or higher heart failure (73.2% vs. 24.9%), but sacubitril/valsartan was again found to be well tolerated.
Across all stratifications evaluated, including those for gender, race, HF history, age, renal function, NYHA class, or baseline NT-proBNP levels, there was a consistency of relative benefit for sacubitril/valsartan over enalapril, supporting the in-hospital initiation of this therapy as a new standard once patients with HFrEF are hemodynamically stable. According to the data presented by Dr. Morrow, neurohormones associated with HF progression remain highly elevated during this period and are likely to explain the substantial vulnerability to adverse HF-related events.
“In the post-PIONEER world, we can keep it simple.”
The PIONEER-HF data were first presented at the 2018 American Heart Association (AHA). At that time, the AHA-invited discussant, Dr. Larry A. Allen, Division of Cardiology, University of Colorado School of Medicine, Aurora, emphasized the advantages of starting hospitalized HFrEF patients on the therapy that they will remain on after discharge rather than transitioning after discharge to sacubitril/valsartan. “In the post-PIONEER world, we can keep it simple,” Dr. Allen said.
In providing the results of the PIONEER-HF extension study, Dr. DeVore made a similar point. He indicated that the published results of PIONEER-HF demonstrate that sacubitril/valsartan can be safely initiated in the hospital using simple criteria. The extension study provides evidence that the protective effect of sacubitril/valsartan relative to enalapril begins almost immediately.
Conclusion
A guideline-recommended therapy for HFrEF in the outpatient setting, PIONEER-HF demonstrated that initiation of sacubitril-valsartan in the hospital immediately reduces NT-proBNP levels relative to enalapril alone. This was associated with a reduction in rehospitalization (8.0% vs. 13.8%) in the published study. With newly-available extension data, the fall in a composite endpoint of death, HF hospitalization, and LVAD implantation for sacubitril/valsartan relative to enalapril was observed early and reached 33% by week 12 (HR 0.67; P=0.02). The findings suggest a potential new standard of care.
Clinical Takeaways:
• PIONEER-HF established that sacubitril/valsartan can be safely initiated in HFrEF patients during hospitalization for acute decompensation
• PIONEER-HF Extension Study demonstrates that in-hospital initiation of sacubitril/valsartan yields a 33% (P=0.02) reduction in a composite endpoint of death, HF hospitalization, and LVAD implantation
• The PIONEER-HF protocol for initiating sacubitril/valsartan in-hospital is appropriate in the routine clinical setting, guided by simple criteria for first establishing hemodynamic stability
• Relevant to the early risk of HF-related events following discharge for acute decompensation, in-hospital initiation of sacubitril/valsartan in HFrEF is a potential new standard