Cardiology
European Society of Cardiology (ESC) Congress 2019
Landmark DAPA-HF Trial: New Approach to Treatment of Heart Failure
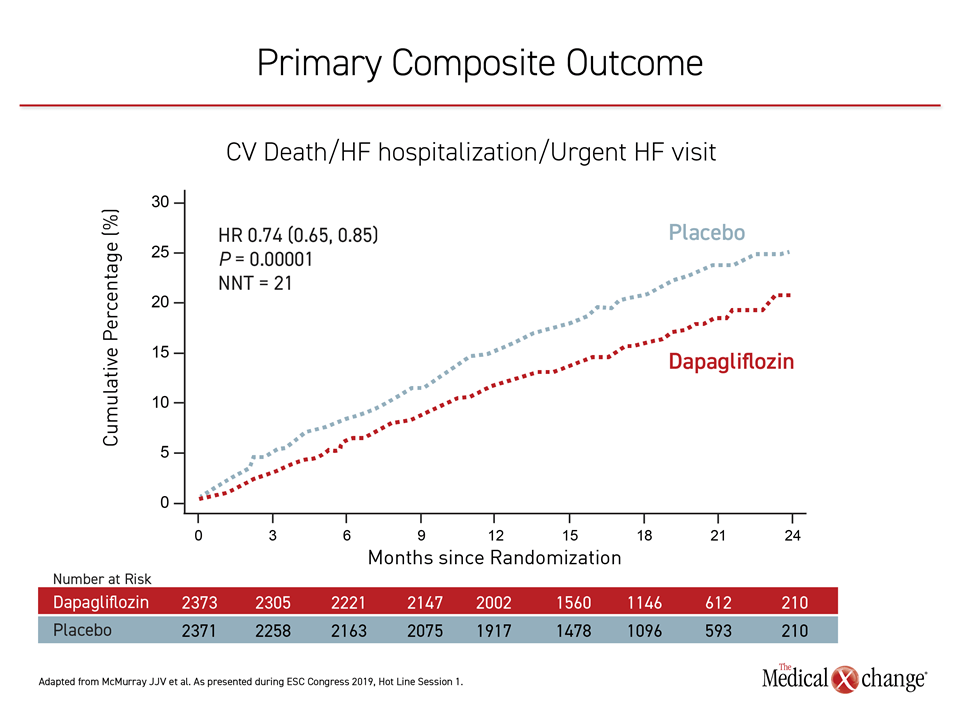
Paris – Standard therapy for heart failure with reduced ejection fraction (HFrEF) has been fundamentally changed by results of a major multinational trial with a sodium-glucose co-transporter 2 (SGLT2) inhibitor. In the randomized placebo-controlled trial, called DAPA-HF, the active therapy on top of standard of care provided a large and significant 26% relative risk reduction (HR 0.74; P=0.00001) in the primary composite outcome of cardiovascular (CV) death, heart failure hospitalization, or urgent heart failure visit requiring intravenous therapy. The degree of benefit, which rivals that seen in any previous trial with a heart failure therapy, was similar whether or not patients had type 2 diabetes mellitus (T2DM).
In DAPA-HF, 4,744 patients with HFrEF from 20 countries including Canada were randomized to 10 mg dapagliflozin or placebo on top of conventional standard HFrEF therapy. Enrollment criteria included a left ventricular ejection fraction (LVEF) ≤40%, an N-terminal pro B-type natriuretic peptide (NT-proBNP) level of ≥600 pg/mL, and New York Heart Association (NYHA) class of II or higher. T2DM was not an entry criterion, but 42% of those enrolled had a prior diagnosis of T2DM and another 3% were identified at entry. The remaining 55% did not have T2DM.
The DAPA-HF trial was called practice changing by numerous experts at the ESC meeting, including principal investigator Dr. John J.V. McMurray, Professor of Cardiology, University of Glasgow, UK.
“This is a new therapy for heart failure with a completely new mechanism of action, which is introducing a new era in heart failure treatment.”
In an unusual reaction when the primary result was displayed during an ESC session for major trials (Figure 1), a sustained ovation from the overflow audience required Dr. McMurray to pause before he could proceed.
“This is a new therapy for heart failure with a completely new mechanism of action, which is introducing a new era in heart failure treatment,” agreed the ESC-invited discussant, Dr. Marco Metra, Head, Division of Cardiology, University of Brescia, Italy. “It is the first time, here, now, that we are showing a benefit from an SGLT2 inhibitor completely independent of diabetes.”
Large and Significant Benefits
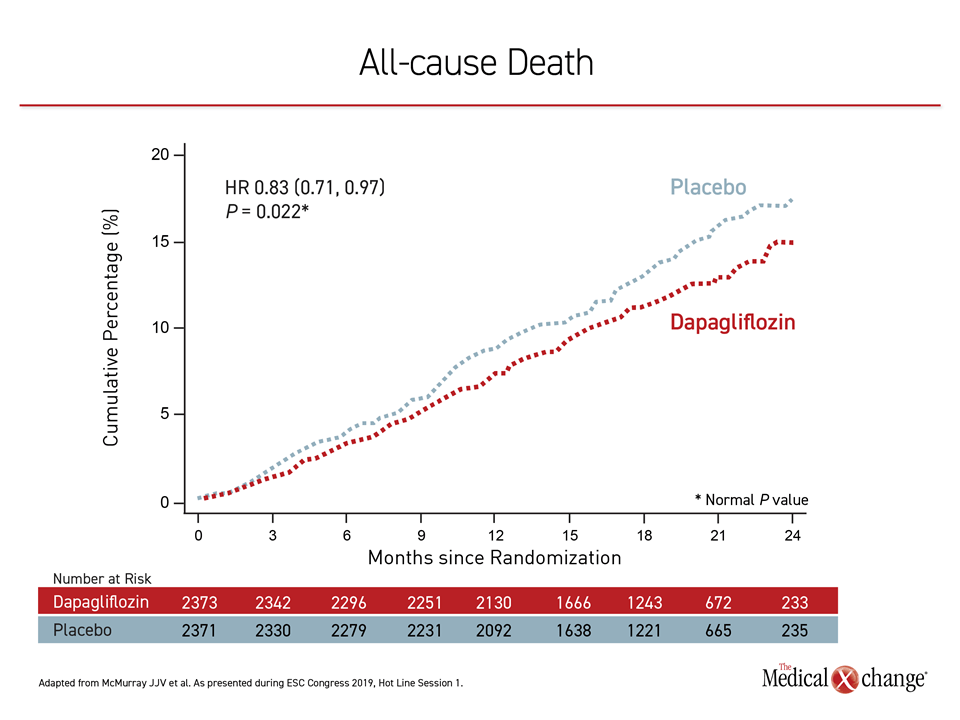
The findings were robust after a median follow-up of 18.2 months. On the endpoint of worsening heart failure alone, which included hospitalizations, the relative risk reduction for dapagliflozin was 30% (HR 0.70; P=0.00003). For CV death, the risk reduction was 18% (HR 0.82; P=0.029). For relative reduction in CV deaths or hospitalization for heart failure, an endpoint employed in several large heart failure trials, including PARADIGM-HF, the risk reduction was 25% (HR 0.75; P=0.0002). A 17% reduction in all-cause mortality was also reached favoring dapagliflozin (HR 0.83; P=0.022) (Figure 2).
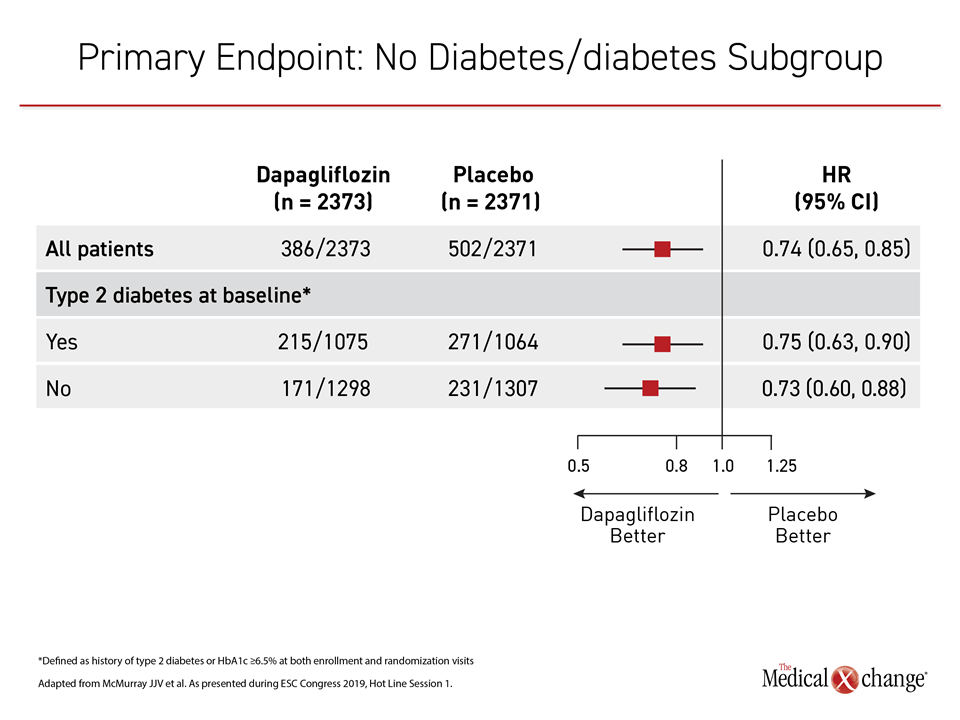
The relative benefit of initiating dapagliflozin in HFrEF was consistent across all 14 prespecified subgroups. The most important of these subgroups from the perspective of a key study hypothesis was the presence or absence of T2DM. For those with diabetes the relative risk reduction in primary endpoint was 25% (HR 0.75, 95% CI 0.63, 0.90). For those without diabetes, the reduction was 27% (HR 0.73, 95% CI 0.60, 0.88) (Figure 3). The relative risk reductions and confidence intervals are not considered meaningfully different.
“All of the benefits [of dapagliflozin] were observed on top of excellent contemporary heart failure therapy.”
The advantage of dapagliflozin over placebo for heart failure-related events could already be seen by the end of the first month on treatment. All of the benefits were observed on top of “excellent contemporary heart failure therapy,” according to Dr. McMurray. The therapies at baseline included a diuretic, an angiotensin-converting enzyme (ACE) inhibitor, angiotensin receptor blocker (ARB), or an angiotensin receptor-neprilysin inhibitor (ARNI) and a beta-blocker in almost all patients. More than 70% of patients were also on a mineralocorticoid receptor antagonist.
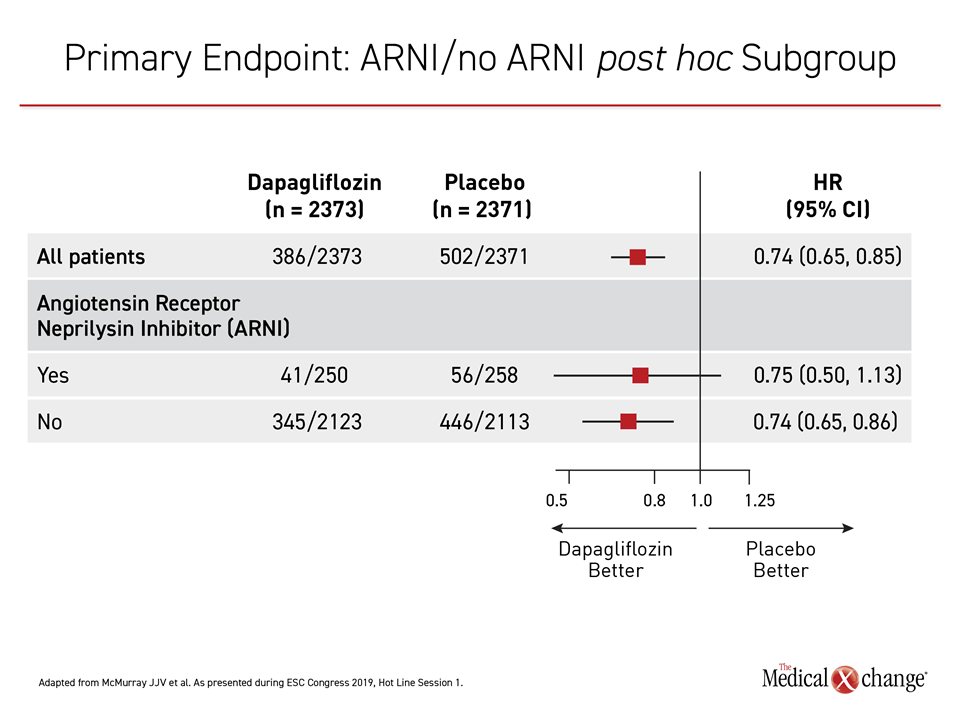
Benefit Seen on Top of ARNI
Only 11% of patients in DAPA-HF were taking an ARNI at baseline because the trial was initiated before the results of PARADIGM-HF made this a standard HFrEF therapy. To consider the potential of this additional therapy to modify the effect of dapagliflozin, an ad hoc analysis was conducted with the DAPA-HF data for this non-prespecified subgroup, according to Dr. McMurray. Again, the advantage of dapagliflozin over placebo was essentially identical for the primary endpoint whether patients were (HR 0.75) or not (HR 0.74) on ARNI therapy (Figure 4).
In this trial, as in previous trials with SGLT2 inhibitors, dapagliflozin proved to be remarkably well tolerated. There was no difference between the active treatment and placebo arms in the overall percentage of patients reporting adverse events. The proportion of patients developing an adverse event leading to discontinuation were comparable between the groups (4.7% vs. 4.9%; P=0.79) and the number of serious adverse events (38 vs. 42; P<0.001) were lower in the group randomized to dapagliflozin.
The reduction in hard events related to heart failure was also accompanied with improvements in quality of life (QoL) as measured with the Kansas City Cardiomyopathy Questionnaire (KCCQ). When assessed eight months into the trial, dapagliflozin showed a 6.1 point increase in KCCQ score from baseline vs. a 3.3 increase in the placebo arm with a statistically significant 2.8 difference between the groups (P<0.001). The proportion of patients with a five-point or greater increase, which is considered clinically meaningful, was significantly greater (58% vs. 51%; P<0.001) and the proportion with a five-point or greater decrease was significantly lower (25% vs. 33%; P<0.001) in the dapagliflozin group.
Heart Failure QoL Benefit Is Significant
“These numbers might not seem impressive for those unfamiliar with these types of data, but I can assure you that these improvements are at least as large as those seen with any prior heart failure therapy,” Dr. McMurray reported.
The importance of these results is that it verifies the benefit “of a new drug with a completely new mechanism” in the control of HFrEF, according to Dr. Metra.
However, the exact mechanism is not yet clear. Some of the early benefit is likely derived from the diuretic action of this SGLT2 inhibitor; however, Dr. Metra cited previous studies that the diuretic effect dissipates over long-term administration. In DAPA-HF, event curves continued to separate over the course of the two-year study. According to Dr. Metra, the persistent benefit might be due to one or more of the purported activities of SGLT2 inhibition, such as improved myocardial metabolism, favorable effects on cardiac ion channels, or beneficial effects on renal function.
“One of the questions for the future is what effect this therapy has on patients with HFpEF,” said Dr. Metra, referring to preserved LV function, typically defined as LVEF ≥50%.
T2DM Agent Effective Without T2DM
The road to a heart failure indication for dapagliflozin in patients without T2DM would have been difficult to predict even a few years ago when the first of the SGLT2 inhibitor cardiac safety trials was published. Initially, these trials, which were mandated by the US Food and Drug Administration, were designed to confirm no increased risk of CV events. Instead and unlike some other types of T2DM therapies, such as thiazolidinediones, the studies with SGLT2 inhibitors have demonstrated CV protection.
Due to the critical importance of reducing CV risk, the most important cause of morbidity and mortality in patients with T2DM, these results have reoriented strategies for glucose lowering that includes overall clinical risk reductions. In patients at the highest risk of CV events, data from EMPA-REG with empagliflozin, CANVAS with canagliflozin, and DECLARE-TIMI 58 with dapagliflozin, all associated the SGLT2 inhibitor with reduction in major adverse cardiovascular events (MACE) that included myocardial infarction (MI) and CV death. However, even in lower risk T2DM patients the protection for heart failure events, including hospitalizations and CV death, was significant. Overall, the reduction in heart failure events in these trials was approximately 30% even though the proportion of patients with heart failure at enrollment was only in the range of 10% to 15%.
From these CV safety trials, “we knew that SGLT2 inhibitors can prevent the development of heart failure in T2DM, but the question we were posing in DAPA-HF is whether they can be used to treat patients with established heart failure,” Dr. McMurray explained.
Benefit Exceeds Expectations
Although a treatment “that both lowers glucose and improves heart failure outcomes is greatly needed” in T2DM, because of the large toll imposed by heart failure in these patients, DAPA-HF tackled a broader hypothesis. The question posed was whether the ability of SGLT2 inhibitors to protect T2DM patients from developing heart failure could prevent events such as hospitalization for HF and CV death, whether or not T2DM was present. The degree of benefit exceeded expectations.
“The relative and absolute risk reductions in death and hospitalization were substantial, clinically important and consistent across all the subgroups we evaluated.”
“The relative and absolute risk reductions in death and hospitalization were substantial, clinically important and consistent across all the subgroups we evaluated,” Dr. McMurray said.
Of the CV safety trials, DECLARE-TIMI 58 was the largest with 17,160 patients. Two-thirds of patients met enrollment criteria on the basis of CV risk factors alone, differing from EMPA-REG, which required a history of CV disease for enrollment, or CANVAS, which also enrolled a high proportion of patients with existing CV disease. Although only about 10% of patients in DECLARE-TIMI 58 had heart failure at entry, a subgroup analysis in the HFrEF population associated dapagliflozin with a 41% (HR 0.59) reduction in all-cause mortality in this population.
DECLARE-TIMI 58 Predicted DAFA-HF
In the HFrEF population of DECLARE-TIMI 58, the relative reduction in the primary endpoint of CV death and hospitalization for heart failure was twice as great (HR 0.62) as that of the trial population overall (HR 0.83). These results predicted the benefit for T2DM patients documented in DAPA-HF, but the equally robust effect in patients without T2DM explain why these results describe a “breakthrough” in HFrEF overall.
“Dapagliflozin does everything we would want a new heart failure drug to do: reduce hospitalization, increase survival, and improve symptoms. These are the three key objectives of treatment,” said Dr. McMurray, who noted that the number needed to treat to achieve the benefit of reduction in worsening of HF and CV death is just 21. “This is the first beneficial heart failure treatment in 10 years not acting through neurohormonal mechanism and the first heart failure treatment to reduce mortality in five years,” he added.
“Dapagliflozin does everything we would want a new heart failure drug to do: reduce hospitalization, increase survival, and improve symptoms.”
In identifying the results of DAPA-HF as establishing a new era in heart failure treatment, Dr. Metra was also referring to the large number of trials with SGLT2 inhibitors that have the potential to further expand their role. This includes the DELIVER trial, which will evaluate dapagliflozin in HFpEF patients, and DAPA-CKD, which is testing the effect of dapagliflozin on renal outcomes and CV mortality in patients with chronic kidney disease. He counted more than 20 ongoing trials using SGLT2 inhibitors.
Conclusion
The landmark DAPA-HF trial has altered standard care in patients with HFrEF. The reduction in the worsening of heart failure-related events was accompanied by a significant reduction in CV death that was not significantly different for those with or without diabetes. The benefit of dapagliflozin was as great or greater as that seen with other now established therapies for heart failure, but was observed on top of these therapies, including an ARNI. The event curves were still diverging at the end of the two-year trial. Experts at the ESC, including the invited discussant, identified these results as practice changing.