Cardiology
American Heart Association (AHA) Scientific Sessions 2019
Large Heart Failure Studies Challenge Definition of Reduced Heart Failure
Philadelphia – The current subcategories of heart failure for guiding pharmacologic therapy are being reconsidered. For patients with chronic heart failure, the Canadian Cardiovascular Society (CCS) treatment guidelines currently make pharmacologic recommendations only in patients with left ventricular ejection fraction (LVEF) of ≤40%, known as heart failure with reduced ejection fraction (HFrEF). Pooled data from two large heart failure trials challenge this cutoff. These data suggest at least some heart failure patients with LVEF >41-49%, which is characterized in the CCS guidelines as heart failure with mid-range ejection fraction (HFmEF), and even patients with LVEF ≥50%, defined as preserved ejection fraction (HFpEF), might also benefit.
New Heart Failure Categories Needed
The debate about how to define appropriate LVEF cutoffs to guide heart failure therapy is not new, but it was intensified by a prespecified pooled analysis from two large studies with an angiotensin receptor neprilysin inhibitor (ARNI). Benefit was observed in heart failure patients with LVEF below and above 40%. Several experts suggested that response at higher LVEF provides additional support for reconsidering heart failure categories and how they are used to guide treatment.
“There appears to be a signal that extends the benefit at least of the ARNI compound in those patients with ejection fractions higher than the current definition,” said Dr. Clyde Yancy, Chief of Cardiology, Feinberg School of Medicine, Northwestern University, Chicago, Illinois. He suggested that this is part of a larger question regarding how to best intervene earlier to prevent heart failure or to more effectively slow progression of deteriorating pump function once it starts.
“There appears to be a signal that extends the benefit at least of the ARNI compound in those patients with ejection fractions higher than the current definition.”
The pooled analysis was from the PARADIGM-HF and PARAGON-HF trials, which together randomized 13,264 patients in 56 countries. The studies employed very similar designs and similar endpoints. A pooled analysis of the two trials before unblinding was prespecified.
PARADIGM-HF compared the ARNI sacubitril/valsartan to the angiotensin-converting enzyme (ACE) inhibitor enalapril in patients with HFrEF at enrollment. For the primary endpoint of time to first event of death from cardiovascular (CV) causes or hospitalization for heart failure, sacubitril/valsartan was associated with a 20% risk reduction (P<0.001) relative to the renin-angiotensin system (RAS) inhibitor. Results of the trial, published in 2014, established sacubitril/valsartan as a standard of care in NYHA class II to IV HFrEF in major guidelines, including those issued by the CCS.
Reconsidering PARAGON-HF Results
In the more recent PARAGON-HF, enrollment was confined to patients with HFpEF, defined as LVEF ≥45%. Patients were randomized to sacubitril/valsartan or valsartan alone. For the primary composite endpoint of total hospitalizations for heart failure or death from CV causes, there was a 13% risk reduction for the ARNI relative to the RAS inhibitor comparator, but this difference narrowly missed statistical significance (P=0.059). In a pre-specified subgroup analysis, the reduction in the primary endpoint was significant for the ARNI relative to valsartan alone (P=0.03) among those who entered the trial below or equal to the mean LVEF of 57%.
The pooled analysis of these two trials was undertaken “to better understand the efficacy of sacubitril/valsartan across the ejection fraction spectrum,” explained Dr. Scott D. Solomon, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts. Presented as a latebreaker at the AHA, these data, while showing efficacy for patients with LVEF ≥45%, led to an extended discussion about the value of the HFmEF category for guiding treatment.
When the data from the two trials was pooled, sacubitril/valsartan was associated with a statistically significant 16% reduction in the primary endpoint of first CV death or heart failure hospitalization when compared to pooled outcomes with a RAS inhibitor.
Benefit Suggested Across LVEF Values
Midway between the 20% reduction in PARADIGM-HF and the 13% reduction in PARAGON-HF, the significant advantage of the ARNI over a RAS inhibitor was observed for the two primary endpoints evaluated individually and for any heart failure hospitalization, which included repeat events. In addition, there was a 12% relative risk reduction in all-cause mortality. All of these risk reductions were statistically significant (95% CI ranging from 0.73 to 0.96).
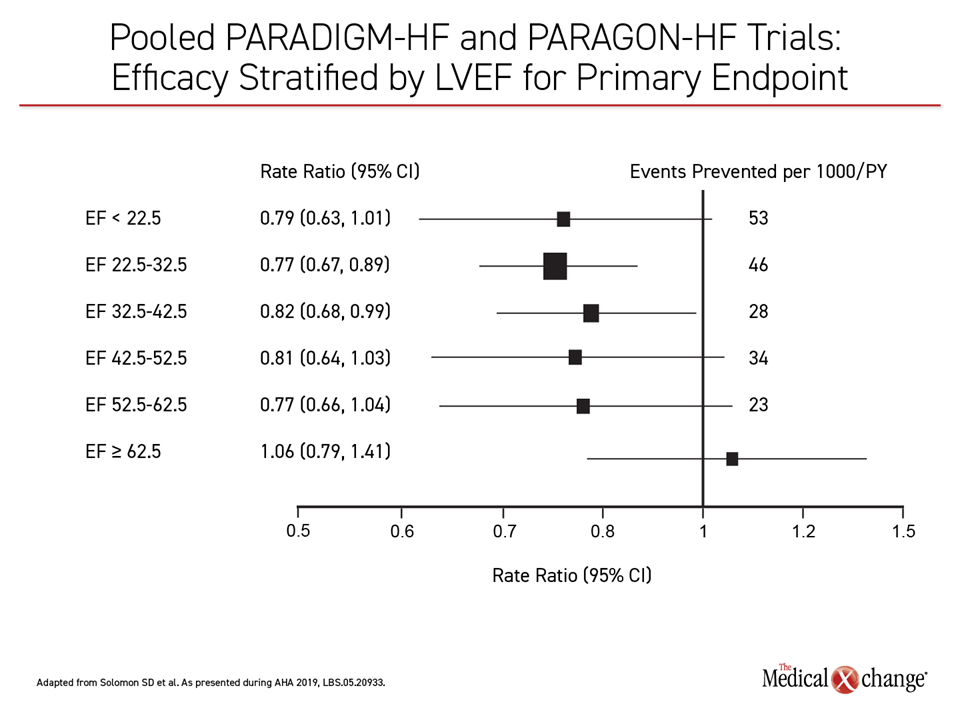
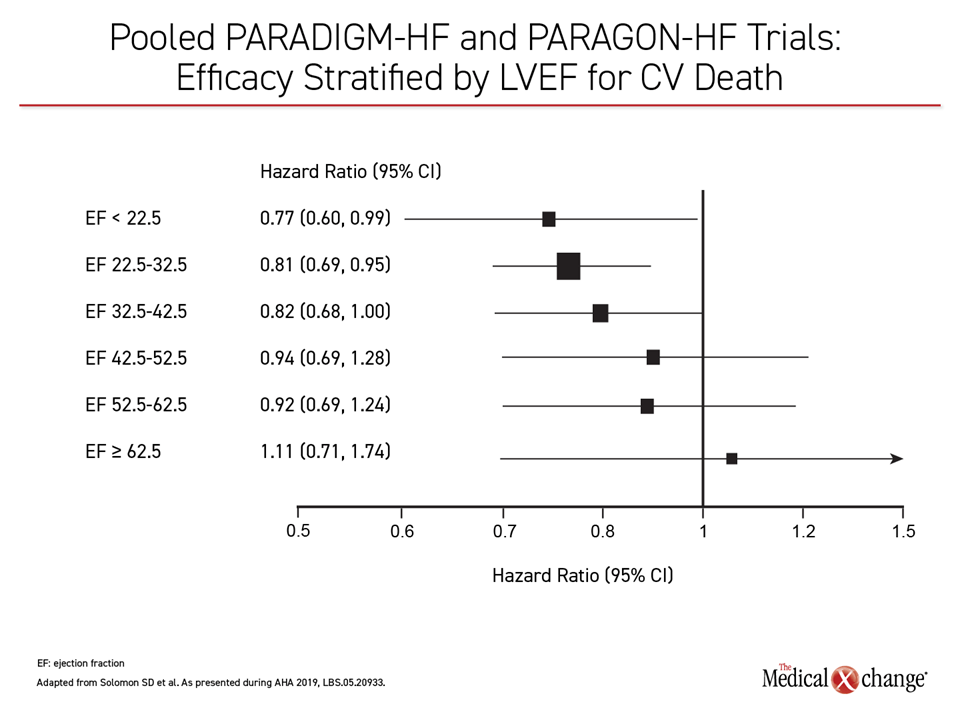
When the primary outcomes were evaluated across five LVEF stratifications ranging from <22.5% to >62.5%, the relative benefit of the ARNI was not confined to the HFrEF population as currently defined. For the combined primary endpoint, the protection provided by the ARNI relative to the RAS inhibitor was surprisingly consistent through the lowest four stratifications (Figure 1). When evaluated as an isolated outcome, protection against CV death was strongest in the lowest three stratifications, but the signal of potential benefit was lost only in the highest stratification (Figure 2).
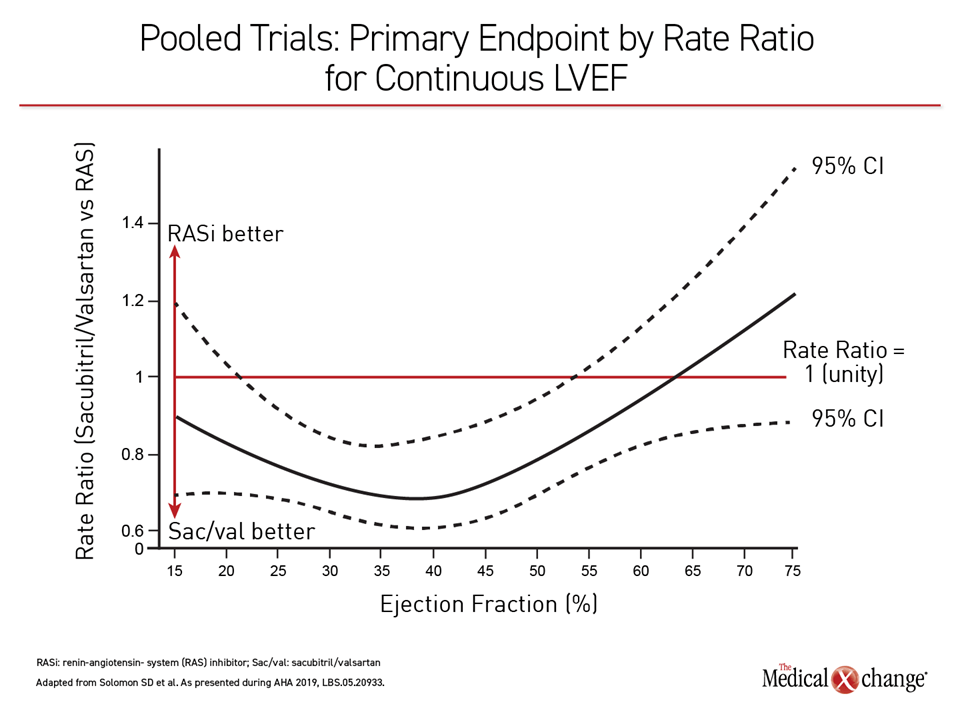
When evaluated as a continuous variable, the combined PARADIGM-HF and PARAGON-HF demonstrate that ARNI benefit occurs on a continuous curve at any single point along the range, rather than that implied by an HFrEF/HFpEF bifurcation. Expressed as a rate ratio, the point at which sacubitril/valsartan no longer provides protection against valsartan alone occurs at an LVEF of approximately 60% (Figure 3).
Women Benefited at Higher LVEF Levels
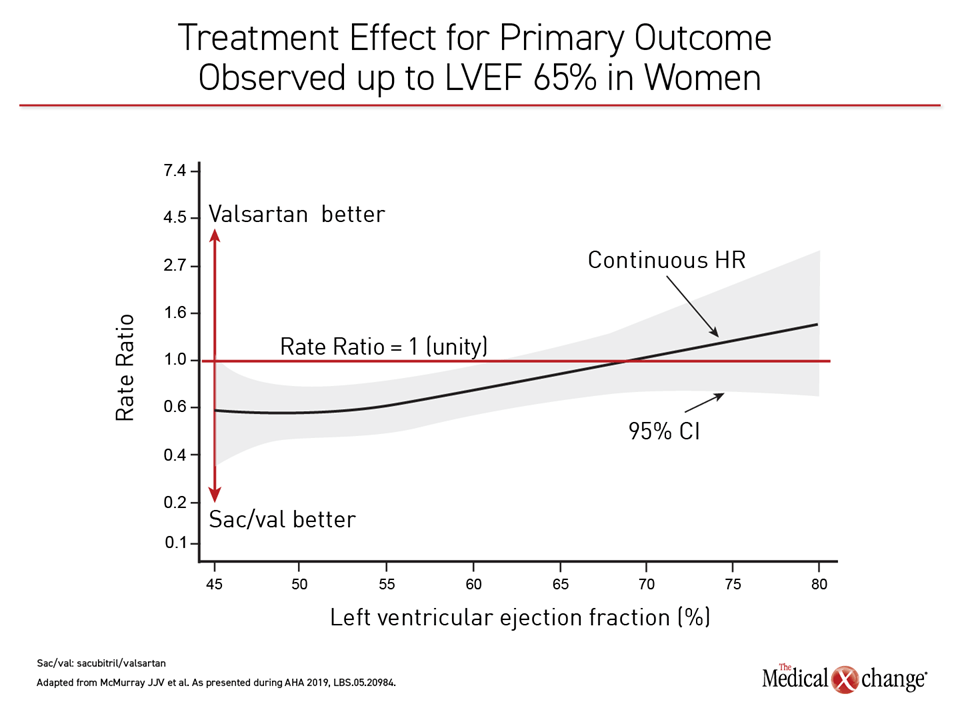
Although the efficacy of sacubitril/valsartan was similar in men and women in the lower ranges of LVEF, the relative benefit of sacubitril/valsartan over a RAS inhibitor persisted at higher levels of LVEF among women. Dr. Solomon said that the reasons are unclear but noted differences between the profiles of men and women with heart failure, including relative older age of onset for women.
Based on these data, “the benefits of sacubitril/valsartan compared to a RAS inhibitor appear to extend to patients with HFmEF with women perhaps benefitting at higher ejection fractions than men,” Dr. Solomon said.
“The benefits of sacubitril/valsartan compared to a RAS inhibitor appear to extend to patients with HFmEF.”
In a separate study and presentation, gender differences were explored in the PARAGON-HF trial. Like the pooled data, this analysis found women might derive particular benefit across a broad range of baseline LVEF values.
Therapeutic Deficit for Female Heart Failure
“Women represent about 25% of patients with HFrEF but more than half of those with HFpEF,” said Dr. John J.V. McMurray, British Heart Foundation Cardiovascular Research Centre, University of Glasgow, UK. He believes this disparity has created a particular “therapeutic deficit” for female heart failure patients.
Of 12 prespecified subgroups in PARAGON-HF, only gender and LVEF appeared to be independent predictors of the primary composite endpoint. In this pre-specified analysis, outcomes were evaluated according to sex.
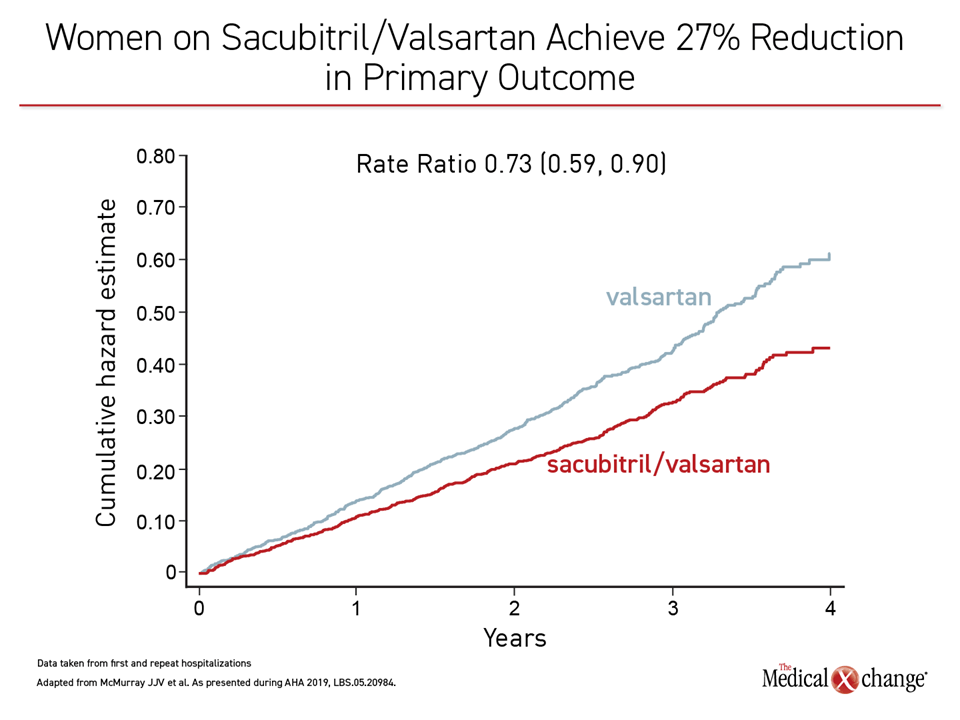
Overall, there was a 27% relative reduction in CV death and total heart failure hospitalizations for sacubitril/valsartan relative to valsartan alone in women, with 95% confidence intervals below the line of unity (Figure 4).
Heart Failure Gender Differences
“These data introduce the possibility that the effects of heart failure drugs in HFpEF might differ between men and women,” Dr. McMurray said. When evaluated across LVEF values within the HFpEF population enrolled in PARAGON-HF, the patterns were different.
“These data introduce the possibility that the effects of heart failure drugs in HFpEF might differ between men and women.”
“In women, there seems to be an advantage for sacubitril/valsartan relative to valsartan in the lower part of the ejection fraction range and then a crossover at about 65%, whereas in men it occurred at a lower LVEF,” Dr. McMurray reported (Figure 5).
Gender Effects Still Uncertain
However, there are arguments both for and against a gender-based difference in response, according to Dr. McMurray. In support of a gender-based difference, he speculated that the greater cardiac remodeling observed in women at high relative levels of LVEF might be more susceptible to neprilysin inhibition. For arguments against concluding there is a gender effect, Dr. McMurray said there is a need for a clearer understanding of an underlying mechanism. He concluded, “Further investigation is warranted.”
Other experts invited to speak about these data also expressed caution in concluding too soon that gender is an important variable in guiding therapy. According to Dr. Lynne W. Stevenson, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, the data presented by Dr. McMurray might relate to disease features that occur more commonly in heart failure patients with relatively well preserved LVEF, including women, but might not be unique to women.
Debate on Gender Differences
“I think it is most likely that the female gender identifies or enhances the selection for certain pathophysiologies that may respond better to some of the peptides that are affected by neprilysin inhibitions,” she said.
“There is indeed a clear signal of benefit [of sacubitril/valsartan] for HFpEF patients in this dataset, but it is a complex signal.”
Dr. Stevenson joined other experts, including Dr. Yancy and Dr. Solomon, in questioning the current strategy of guiding heart failure treatment through the categories of HFrEF or HFpEF alone. She agreed that the pooled data from PARADIGM-HF and PARAGON-HF show that sacubitril/valsartan does provide protection for heart failure-related events in patients with LVEF above the levels for which it is indicated.
“There is indeed a clear signal of benefit for HFpEF patients in this dataset, but it is a complex signal,” said Dr. Stevenson. However, she argued that the goal is not just creating new names for heart failure severity based on LVEF but pursuing more data about differentiating key therapeutic targets at different points in disease.
Conclusion
Pooled data from the PARADIGM-HF and PARAGON-HF data suggest that the relative advantage of sacubitril/valsartan over valsartan alone in preventing heart failure-related events occurs along an LVEF continuum that exceeds the current guideline definition of HFrEF. A benefit in patients with LVEF >40% was observed in both men and women with heart failure, but both the pooled data and a prespecified PARAGON-HF subanalysis indicate that women may derive benefit at even higher levels of LVEF. The results challenge current guidelines for heart failure of mid-range or preserved ejection fraction.
Clinical Takeaways:
• In pooled data from PARADIGM-HF and PARAGON-HF, there was a significant benefit from sacubitril/valsartan over valsartan in patients with NYHA class II heart failure over a range of LVEF levels including those that fall into the HFrEF and HFpEF categories.
• At higher ranges of LVEF, the relative benefit of sacubitril/valsartan over valsartan alone appeared to be greater in women than men.
• The mechanism underlying the gender differences in response to sacubitril/valsartan in patients with higher levels of LVEF are unknown but may be related to disease features that are more common in women than men.
• The pooled data from PARADIGM-HF and PARAGON-HF contribute to an ongoing debate about the potential need for considering a heart failure with mildly reduced ejection fraction (HFmEF) to bridge a gap between established treatments for HFrEF and less severe disease.