Dermatology
CSU: Expert Review and Commentary from Published Literature
Chronic Spontaneous Urticaria Is Not an Allergic Process: The Limited Value of Extensive Testing
Harold Kim, MD, FRCPC
Adjunct Professor, Division Chair/Chief, Clinical Immunology and Allergy, Western University
Assistant Clinical Professor, McMaster University
President, Canadian Society of Allergy and Clinical Immunology
Kitchener, Ontario
In patients with the clinical features of chronic urticaria, diagnostic tests, including skin tests for allergens, are not a standard of care. Rather, the diagnosis of chronic urticaria can and should be based primarily on history and physical examinations. When the disease presentation suggests etiologies other than urticaria, further testing is appropriate, but extensive investigations or screening for triggers, such as allergens in a patient with classic features have a low yield and delay treatment. In patients with chronic urticaria, the primary goal is to promptly initiate the evidence-based treatment algorithm in order to restore an acceptable quality of life.
Chronic urticaria is defined by persistent or recurrent symptoms over a period greater than six weeks. The inducible forms, such as cold or cholinergic urticaria, represent a minority of cases. The more common diagnosis is chronic spontaneous urticaria (CSU). Once known as idiopathic urticaria because the exact etiology is unknown, CSU can be reliably differentiated from inducible forms as well as from alternative conditions on the basis of history and physical. Conversely, extensive testing is unlikely to alter initial treatment or, in the majority of cases, identify an avoidable trigger.
CSU can and should be diagnosed in the primary care setting, where the initiation of first-line therapies is appropriate. Specialist referrals for CSU patients inadequately controlled on first-line therapies should be for the purpose of advanced care instead of additional testing.
Background
Urticaria, more commonly known as hives, involves pruritic wheals typically accompanied by erythema and edematous papules. In chronic urticaria, the wheals are accompanied by angioedema in approximately 40% of cases.2 Unlike acute urticaria, which might resolve within hours but can last days or weeks, urticaria is considered chronic when symptoms persist or recur for a period of six weeks or longer.1 At a lifetime prevalence of 15% to 20% and a point prevalence of 0.5% to 1.0%,3 chronic urticaria is a relatively common complaint.
For an eLearning program with Dr. Harold Kim on the impact to clinical practice, click here
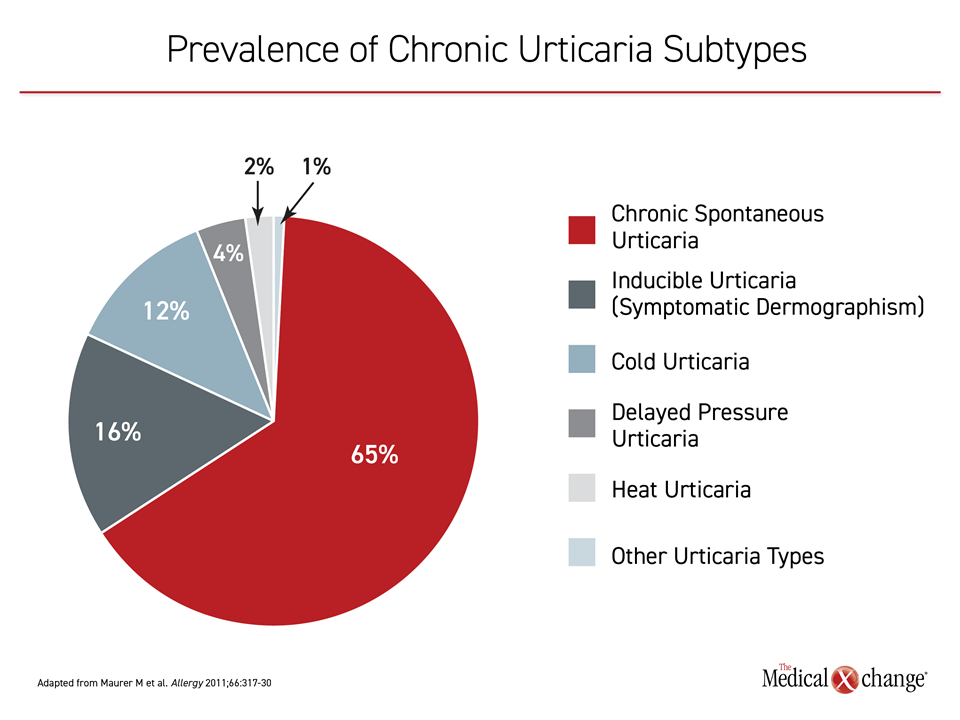
Of chronic urticaria subtypes, CSU is the most common, accounting for up to 65% of cases3 (Figure 1). Unlike the inducible forms, such as symptomatic dermographism or cholinergic urticaria, patients with CSU are less likely to consistently associate the onset of symptoms with a specific trigger. There is now a growing body of evidence suggesting that many cases of CSU involve an autoimmune process resulting in mast cell activation, histamine release, and wheal formation.4
CSU can occur at any age, but the peak prevalence occurs between the ages of 20 and 40 years.3 There appears to be a slightly higher prevalence in women than men.5 Wheals, which are typically 1 to 3 mm in size but sometimes coalesce to form larger lesions, can occur on any skin surface and are typically more itchy than painful. In those with burning and painful lesions, evaluation for urticarial vasculitis, a potentially serious disorder, is warranted.
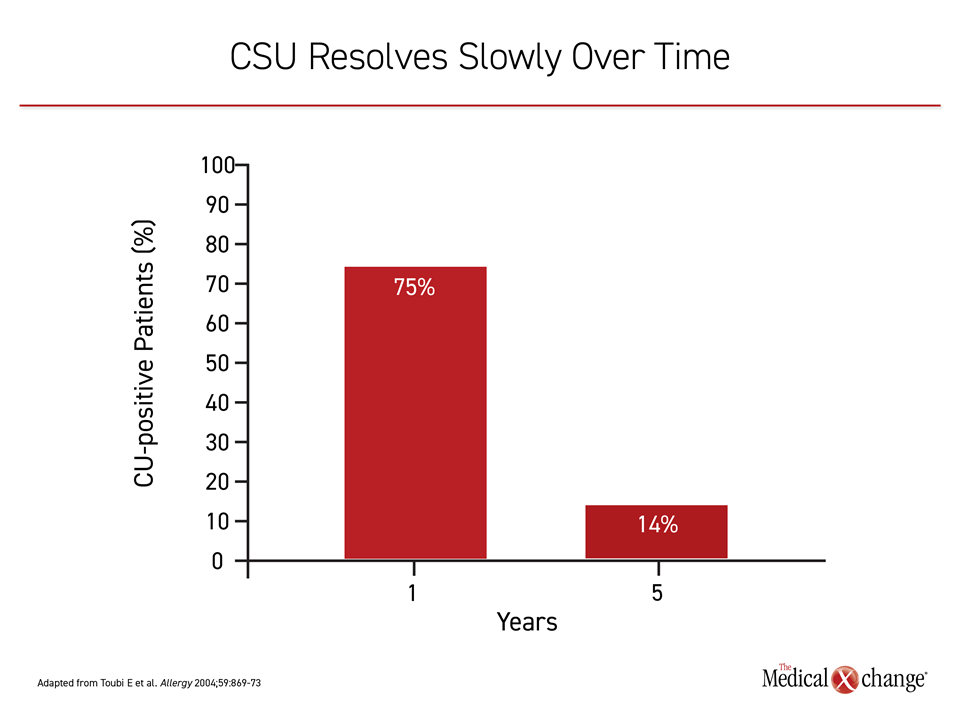
The duration of CSU is highly variable. In one study, symptoms had resolved at one year in 35% of patients and diminished in nearly half of the remaining.6 In another, which followed patients for five years, 70% continued to experience symptoms off treatment at the end of one year, but there was a progressive decline over time.7 Only 14% still experienced symptoms off treatment at five years (Figure 2).
CSU imposes a major adverse impact on quality of life. In addition to the discomfort of persistent, itchy and sometimes painful lesions, which can interfere with sleep and next-day productivity,8 the cutaneous manifestations can cause embarrassment.9 These issues might explain the association between chronic urticaria and increased risk of depression and anxiety.10 CSU is non-life-threatening, but the burden of this condition encourages prompt diagnosis and appropriate intervention.
Pathophysiology: Why Not Look for Triggers?
In treatment guidelines for CSU, extensive diagnostic testing is discouraged. One reason is that the yield is low. On the basis of history, suspected triggers are helpful for guiding therapy that includes avoidance strategies, but provocation testing not suggested by patient history is not required and should not be routinely performed. In general, symptoms controlled by trigger avoidance provide an empirical confirmation of a contributing etiological factor, but testing for triggers consumes resources with a low likelihood of identifying a precipitating exposure. No definitive etiology can be identified in the majority of cases of CSU.11
Basic laboratory tests, such as a complete blood count (CBC) with differential and C-reactive protein (CRP) are recommended in the current guidelines as part of a rule-out process that inform the history and physical.1 While evidence of systemic disease, such as an elevated CRP, warrants additional investigation, there is a low likelihood of an alternative diagnosis in patients with skin lesions consistent with CSU but no other health issues.12 It is important to recognize that those patients who do have an underlying systemic disease, including relatively rare diseases, such as Schnitzler’s syndrome and cryopyrin-associated periodic syndrome (CAPS),13,14 will have symptoms beyond those involving the skin.
Urticaria of any form, whether acute or chronic, inducible or spontaneous, is typically driven by degranulation of mast cells and basophils in the skin. When IgE receptors on the surface of these cells are triggered, the activated cells release histamine and proinflammatory mediators, such as interleukins-4 (IL-4), IL-5, and IL-8, and platelet activating factor (PAF).15 Angioedema occurs when mast cell activation occurs deeper or lower in the dermis or subcutis.16 Mast cells are considered a critical participant in the inflammatory response that drives urticaria, but other cells, including lymphocytes, and mediators other than histamine, such as leukotrienes, participate in some urticarial lesions.
For inducible chronic urticaria, the mast cell activation is provoked by a specific exposure. Some inducible urticaria involves type 1 reactions that occur when allergens bind to cell surface antibodies, but physical triggers produce many without IgE activation. For CSU, the trigger remains unclear and might vary among individuals. One prominent theory is that dysregulation of intracellular signaling with or without an increased susceptibility or hyperresponsiveness leads to mast cell activation.4 Another suggests an autoimmune pathology in which circulating autoantibodies trigger an inflammatory response that involves histamine release.17
Theories of Pathogenesis Are Incomplete
Neither of the theories of pathogenesis has been confirmed or so far proven helpful in the diagnosis or treatment of CSU. If there is a disturbance in intracellular signaling, it is unclear why it develops or why it typically resolves over time. Although studies associating autoantibodies and chronic urticaria date back to the early 1990s,18 these associations have been relative rather than absolute and no prognostic or therapeutic value has been derived from demonstrating specific autoantibodies.19
In practical terms, the diagnosis of CSU is an empirical process based on exclusion. Although there are no biomarkers or definitive tests to establish CSU, patients can be reassured of the diagnosis when symptoms are skin related in the absence of systemic involvement. Symptom improvement with first-line antihistamines provides additional evidence of mast cell activation and underlying CSU.
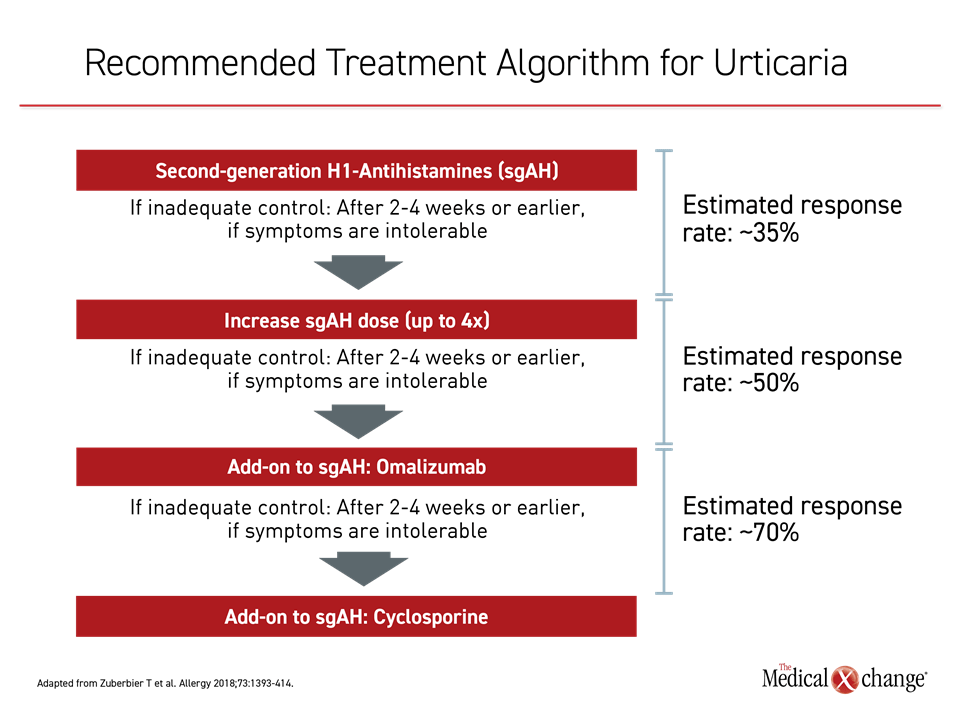
With three simple guideline-recommended steps, the vast majority of patients with CSU achieve adequate symptom control. The first step is standard-dose antihistamine (Figure 3). According to the guidelines, second-generation antihistamines, which are better tolerated than the earlier generation products, are preferred. In CSU patients, continuous therapy is more beneficial, particularly in regard to measures of quality of life, than as-needed treatment.20 The head-to-head comparative studies of available second-generation antihistamines for urticaria have suggested potential differences, but any of the currently available agents with low potential for drug-drug interactions are considered appropriate, according to the guidelines.1
In patients with inadequate control of symptoms, dose escalation of the antihistamine is the next step. Several upward titrations can be offered when needed. The guidelines recommend intervals of two to four weeks but more rapid upward titrations can be offered if symptoms are intolerable. Up to four times the standard dose is recommended. By the time that the antihistamine dose has been maximized to the recommended limit, approximately half of patients with CSU can expect to achieve adequate symptom control.12
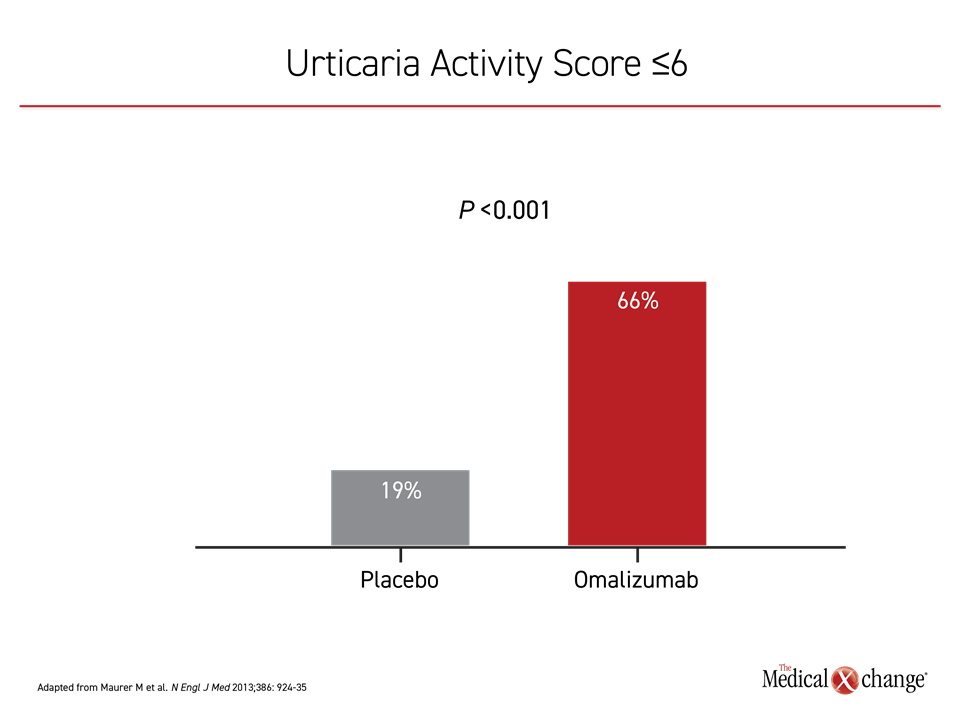
The targeted anti-IgE monoclonal antibody omalizumab represents the third step in the treatment algorithm. By inhibiting IgE signaling, omalizumab blocks mast cell and basophil activation.21 In a placebo-controlled phase 3 trial, the highest evaluated dose of 300 mg reduced the mean itch-severity score at 12 weeks by more than 70% relative to baseline and by 60% relative to placebo.22 The reduction in hives was of similar magnitude. When compared for symptom control, defined as Urticaria Activity Score (UAS) of 6 or less, the difference at 12 weeks was highly significant (P<0.001). (Figure 4) These symptom reductions were reflected in improvements in the Dermatology Life Quality Index (DLQI). The adverse event profile was comparable to placebo.
With the addition of a standard dose of omalizumab, which is 300 mg administered subcutaneously every four weeks, adequate symptom control is achieved in approximately 70% to 80% of patients. For the minority of patients who do not respond to standard-dose omalizumab, the guidelines recommend cyclosporine, which has also been associated with symptomatic improvement in placebo-controlled trials.23,24 However, the risk of adverse events increases over time. Dosing of more than several weeks requires monitoring for effects on blood pressure and kidney function, and use for longer than six months should be undertaken with caution.25
Corticosteroids, although potentially effective for short-term symptom control, are strongly discouraged in the guidelines as a CSU maintenance therapy. In many countries, corticosteroids must be used off-label. The guidelines recommend a treatment course of no longer than 10 days.
Treatment guidelines commonly list drugs used empirically in CSU, such as dapsone, methotrexate, phototherapy, intravenous immunoglobulins and leukotriene inhibitors, but none have been shown effective in controlled trials.1 Similarly, there are case reports of improved response when higher or more frequent doses of omalizumab are used in those inadequately controlled on a standard dose, but controlled trials are needed.26
For refractory patients, experimental treatments are showing promise. Of agents in clinical trials, a next-generation anti-IgE antibody has reached advanced stages of clinical testing by showing high rates of efficacy and acceptable safety. In a phase 2b dose-finding trial with this agent, called ligelizumab, 382 CSU patients inadequately controlled on high-dose antihistamines were randomized to 300 mg omalizumab, placebo, or to one of three ligelizumab doses administered by subcutaneous injection every 4 weeks.27 The primary endpoint was complete control of hives at 12 weeks. On this relatively rigorous definition of symptom control, 51% of patients on the most effective dose of ligelizumab but only 26% of those in the omalizumab group and none of those randomized to placebo met the endpoint. Based on these findings, a phase 3 trial is anticipated.
CSU: The Practical Approach
Despite the many unanswered questions about CSU, which includes uncertainties about the core events that drive this disease, this is not necessarily a challenging condition for diagnosis and initial treatment in the primary care setting. Chronic urticaria is defined by persistent hives in the absence of a causative systemic disease as evaluated with history and physical. If symptoms resolve when potential triggers are identified on history, an inducible form is confirmed. When no triggers are identified or if trigger avoidance is not effective, improvement on an antihistamine is reassuring for the underlying diagnosis of CSU.
Diagnostic studies beyond a history or physical are discouraged. They threaten prompt treatment with limited potential to improve treatment choices or outcomes. Accelerating the time to treatment is an important goal. In an international cohort observational study of patients with CSU, about half had moderate-to-severe disease activity as measured by symptoms scores or impact on quality of life.9 The same study associated CSU with a major adverse impact on quality of life and activities of daily living, including sleep and work performance. Of the 72% of patients who had a CSU-related healthcare visit in the previous 12 months, the average number of visits was 3.3. CSU-related hospitalizations were less common, recorded in 8.2% of patients, but the estimated societal economic burden from direct and indirect costs were substantial.
Diagnostic studies beyond a history or physical are discouraged. They threaten prompt treatment with limited potential to improve treatment choices or outcomes.
In a chicken-and-egg dilemma, it is unclear whether the high rate of comorbidities is a complication or consequence of CSU. In addition to an increased rate of autoimmune comorbidities, such as Graves’ disease, vitiligo, and rheumatoid arthritis than would be expected in the general population,28 it has been postulated that the higher rates of depression and anxiety associated with CSU stem from stress that can both trigger and exacerbate the disease.29 The role of stress in causing CSU, rash, or itching remains controversial despite the frequency with which these associations are made empirically, but the evidence of a brain-gut-skin axis support a neurogenic component to potentially related comorbidities.30
The 2017 guidelines do not discuss the risk or strategies for managing the comorbidities of CSU, but future guidelines are likely to address this issue as a result of an increase in published studies in this area. Psychiatric disorders, for example, are commonly observed in patients with CSU.31 Fatigue and gastrointestinal complaints are also commonly associated with CSU.30 It is reasonable to inquire about comorbidities in a comprehensive management plan although the ability of treatments effective for CSU to diminish the presence or severity of comorbidities has not been well evaluated.
Clear evidence-based guidelines for treatment are appropriately applied in the primary care setting. Antihistamines, the first-line treatment, are effective and well tolerated even if uptitration is needed for symptom control. It is expected that not all primary care clinicians will be comfortable prescribing or administering omalizumab, the next step when high-dose antihistamines fail, but referral should not be delayed. There have been no significant safety concerns with this agent since it entered widespread clinical use more than five years ago. Most CSU patients initiated on this agent will achieve adequate symptom control for an otherwise debilitating disease.
It is expected that not all primary care clinicians will be comfortable prescribing or administering omalizumab, the next step when high-dose antihistamines fail, but referral should not be delayed.
For the minority of patients who do not respond to the first three steps on guideline-recommended therapy, the next option is more problematic. Cyclosporine, which is recommended, is associated with significant side effects and is best employed judiciously over limited periods of time. The immunomodulating agents beyond these therapies are largely uninvestigated and employed empirically.
Despite its substantial prevalence, CSU remains inadequately recognized and treated at the primary care level. Once difficult to control in patients without an adequate response to antihistamines, the IgE-targeted omalizumab increases likelihood of treatment success. Biomarkers may eventually provide insight on the pathology or pathologies of CSU to yield objective diagnostic tests and new therapeutic strategies, but the current guidelines for identifying patients with CSU and initiating treatment are simple and effective in most patients.
Summary
CSU is not a life-threatening condition but can impose a meaningful reduction in quality of life even when present in mild to moderate severity. The diagnosis can be presumed in patients with characteristic skin lesions and pruritus in the absence of signs of a significant systemic disease. On antihistamines, the first-line therapy, most patients will achieve adequate symptom control following a treatment algorithm that includes high doses of second-generation antihistamines and, if needed, an anti-IgE monoclonal antibody. The goal of treatment is complete resolution of symptoms. Over time, CSU resolves in most patients although persistent disease is observed in a minority of individuals.
References
1. Zuberbier T, Aberer W, Asero R, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy 2018;73:1393-414.
2. Kaplan AP. Clinical practice. Chronic urticaria and angioedema. N Engl J Med 2002;346:175-9.
3. Maurer M, Weller K, Bindslev-Jensen C, et al. Unmet clinical needs in chronic spontaneous urticaria. A GA(2)LEN task force report. Allergy 2011;66:317-30.
4. Bracken SJ, Abraham S, MacLeod AS. Autoimmune Theories of Chronic Spontaneous Urticaria. Front Immunol 2019;10:627.
5. Eun SJ, Lee JY, Kim DY, Yoon HS. Natural course of new-onset urticaria: Results of a 10-year follow-up, nationwide, population-based study. Allergol Int 2019;68:52-8.
6. Kozel MM, Mekkes JR, Bossuyt PM, Bos JD. Natural course of physical and chronic urticaria and angioedema in 220 patients. J Am Acad Dermatol 2001;45:387-91.
7. Toubi E, Kessel A, Avshovich N, et al. Clinical and laboratory parameters in predicting chronic urticaria duration: a prospective study of 139 patients. Allergy 2004;59:869-73.
8. Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol 2014;134:1527-34.
9. Maurer M, Abuzakouk M, Berard F, et al. The burden of chronic spontaneous urticaria is substantial: Real-world evidence from ASSURE-CSU. Allergy 2017;72:2005-16.
10. Tat TS. Higher Levels of Depression and Anxiety in Patients with Chronic Urticaria. Med Sci Monit 2019;25:115-20.
11. Schaefer P. Acute and Chronic Urticaria: Evaluation and Treatment. Am Fam Physician 2017;95:717-24.
12. Kaplan AP. Chronic Spontaneous Urticaria: Pathogenesis and Treatment Considerations. Allergy Asthma Immunol Res 2017;9:477-82.
13. Davis MDP, van der Hilst JCH. Mimickers of Urticaria: Urticarial Vasculitis and Autoinflammatory Diseases. J Allergy Clin Immunol Pract 2018;6:1162-70.
14. Keddie S, Parker T, Lachmann HJ, Ginsberg L. Cryopyrin-Associated Periodic Fever Syndrome and the Nervous System. Curr Treat Options Neurol 2018;20:43.
15. Jain S. Pathogenesis of chronic urticaria: an overview. Dermatol Res Pract 2014;2014:674709.
16. Champion RH, Roberts SO, Carpenter RG, Roger JH. Urticaria and angio-oedema. A review of 554 patients. Br J Dermatol 1969;81:588-97.
17. Asero R, Tedeschi A, Riboldi P, Cugno M. Plasma of patients with chronic urticaria shows signs of thrombin generation, and its intradermal injection causes wheal-and-flare reactions much more frequently than autologous serum. J Allergy Clin Immunol 2006;117:1113-7.
18. Grattan CE, Francis DM, Hide M, Greaves MW. Detection of circulating histamine releasing autoantibodies with functional properties of anti-IgE in chronic urticaria. Clin Exp Allergy 1991;21:695-704.
19. Bernstein JA, Lang DM, Khan DA, et al. The diagnosis and management of acute and chronic urticaria: 2014 update. J Allergy Clin Immunol 2014;133:1270-7.
20. Grob JJ, Auquier P, Dreyfus I, Ortonne JP. How to prescribe antihistamines for chronic idiopathic urticaria: desloratadine daily vs PRN and quality of life. Allergy 2009;64:605-12.
21. Beck LA, Marcotte GV, MacGlashan D, Togias A, Saini S. Omalizumab-induced reductions in mast cell Fce psilon RI expression and function. J Allergy Clin Immunol 2004;114:527-30.
22. Maurer M, Rosen K, Hsieh HJ, et al. Omalizumab for the treatment of chronic idiopathic or spontaneous urticaria. N Engl J Med 2013;368:924-35.
23. Grattan CE, O’Donnell BF, Francis DM, et al. Randomized double-blind study of cyclosporin in chronic ‘idiopathic’ urticaria. Br J Dermatol 2000;143:365-72.
24. Vena GA, Cassano N, Colombo D, Peruzzi E, Pigatto P, Neo ISG. Cyclosporine in chronic idiopathic urticaria: a double-blind, randomized, placebo-controlled trial. J Am Acad Dermatol 2006;55:705-9.
25. Koski R, Kennedy KK. Treatment with omalizumab or cyclosporine for resistant chronic spontaneous urticaria. Ann Allergy Asthma Immunol 2017;119:397-401.
26. Clark JJ, Secrest AM, Hull CM, et al. The effect of omalizumab dosing and frequency in chronic idiopathic urticaria: Retrospective chart review. J Am Acad Dermatol 2016;74:1274-6.
27. Maurer M, Gimenez-Arnau AM, Sussman G, et al. Ligelizumab for Chronic Spontaneous Urticaria. N Engl J Med 2019;381:1321-32.
28. Kolkhir P, Borzova E, Grattan C, Asero R, Pogorelov D, Maurer M. Autoimmune comorbidity in chronic spontaneous urticaria: A systematic review. Autoimmun Rev 2017;16:1196-208.
29. Bansal CJ, Bansal AS. Stress, pseudoallergens, autoimmunity, infection and inflammation in chronic spontaneous urticaria. Allergy Asthma Clin Immunol 2019;15:56.
30. Aitella E, De Bartolomeis F, Savoia A, Fabiani M, Romano M, Astarita C. The overlap syndrome of urticaria and gastroesophageal reflux disease. PLoS One 2018;13:e0207602.
31. Konstantinou GN, Konstantinou GN. Psychiatric comorbidity in chronic urticaria patients: a systematic review and meta-analysis. Clin Transl Allergy 2019;9:42.