Dermatology
GA2LEN UCARE Conference 2019: Expert Review
Adequate Care across the Spectrum of Chronic Urticaria is in the Process of Redefinition
Davindra Singh, BA, MD, CCFP (EM), FRCPC
Founder & Lead Dermatologist, AvantDerm
Toronto, Ontario
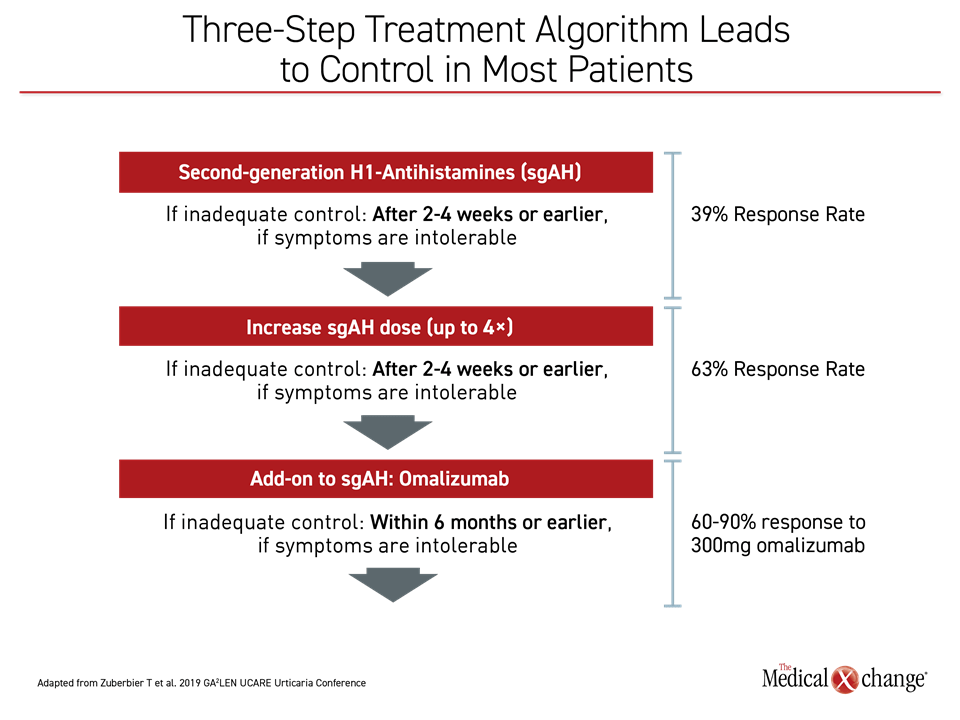
Deliberations for new urticaria guidelines will begin at the end of 2020. Superseding the EAACI/ GA2LEN/EDF/WAO guidelines published in 2018,1 these are expected to include new information on comorbidities, urticarial subtypes, and biomarkers, all of which were updated at the 2019 Global Allergy and Asthma European Network (GA2LEN) / Urticaria Centers of Reference and Excellence (UCARE) Urticaria Conference. Ongoing clinical trials of new therapies might also be completed in time for the coming guidelines. Although most patients with chronic spontaneous urticaria (CSU) respond to the currently recommended three-step treatment algorithm, data presented here suggest that coming therapies will produce control in most patients with chronic urticaria not currently controlled on available options.
Background
Since completion of the previous guidelines and the first GA2LEN UCARE Urticaria Conference held in Barcelona in 2017, the progress in understanding, classifying, and treating chronic urticaria has been substantial. Summarized at the 2019 GA2LEN UCARE Conference, this is credited to the increasingly systematic approach fostered by the UCARE Network, a global collaboration, as well as to the real-world data accruing in the Chronic Urticaria Registry (CURE) (Figure 1).
For an exclusive interview with Dr. Davindra Singh on the impact to clinical practice, click here
The UCARE network already has 91 participating centers in 36 countries and remains on a steep growth trajectory since its formation three years ago, according to Dr. Marcus Maurer, Director of Research for the Department of Dermatology and Allergy, Charité University of Medicine, Berlin, Germany, and member of the GA2LEN UCARE Urticaria Conference Scientific and Organizing Committee. The growing membership and participation in the many UCARE initiatives has been critical for driving research. The ultimate goal is ambitious.
“It is nice to give our patients therapies to make their itch go away, but this is not where we want to be in 10 years. We need to be thinking how to get to a cure,” said Dr. Maurer.
Over the two-day conference, which attracted participants from more than 40 countries, key themes included advances in treatment for non-responders to standard options, the management of comorbidities, and the emerging strategies to monitor disease, including patient-reported outcomes (PROs) and biomarkers. Near the start of the meeting, data were updated from CURE, a global patient registry that will be an increasingly powerful source of data on chronic urticaria as enrollment accrues.
“It is nice to give our patients therapies to make their itch go away, but… we need to be thinking how to get to a cure.”
CURE was initially launched in Germany but opened to worldwide participation in 2016. Currently, 36 centers in 22 countries are providing data, but participation has been on a steep growth curve both in regard to institutions enrolling and patient data submitted. There are now nearly 3000 patients in this database. To track a condition with many subtypes and a variable course, CURE is considered an essential tool for better characterization of chronic urticaria.
“Clinical studies of chronic urticaria can only partly represent the real-world experience because of the heterogeneity and many subtypes of urticaria,” reported Dr. Karsten Weller, a director of CURE and a colleague of Dr. Maurer’s in the Charité Department of Dermatology and Allergy. “These data can permit us to pose questions about urticaria that would be difficult to address in randomized controlled trials.”
In a sample of CURE data summarized by Dr. Weller, 57% of the chronic urticaria patients entered into the registry so far presented with wheals only. Of the remaining, 37% had both wheals and angioedema. Just 6% presented with angioedema alone.
“The CURE data can permit us to pose questions about urticaria that would be difficult to address in randomized controlled trials.”
CURE is enrolling patients with CSU or chronic inducible urticaria (CindU). When these two groups were compared, the mean age in the 2066 CSU patients, which represents 76.9% of the patients enrolled to date, were slightly older than the 621 with CindU (45.0 vs. 39.6 years) but the proportion of females was nearly identical (71% vs. 70%). Consistent with previously-published observations, the five most common CindU subtypes were symptomatic dermographism (38.8%), cold urticaria (29.4%), cholinergic urticaria (14.6%), delayed pressure urticaria (9.5%) and heart urticaria (4.7%).
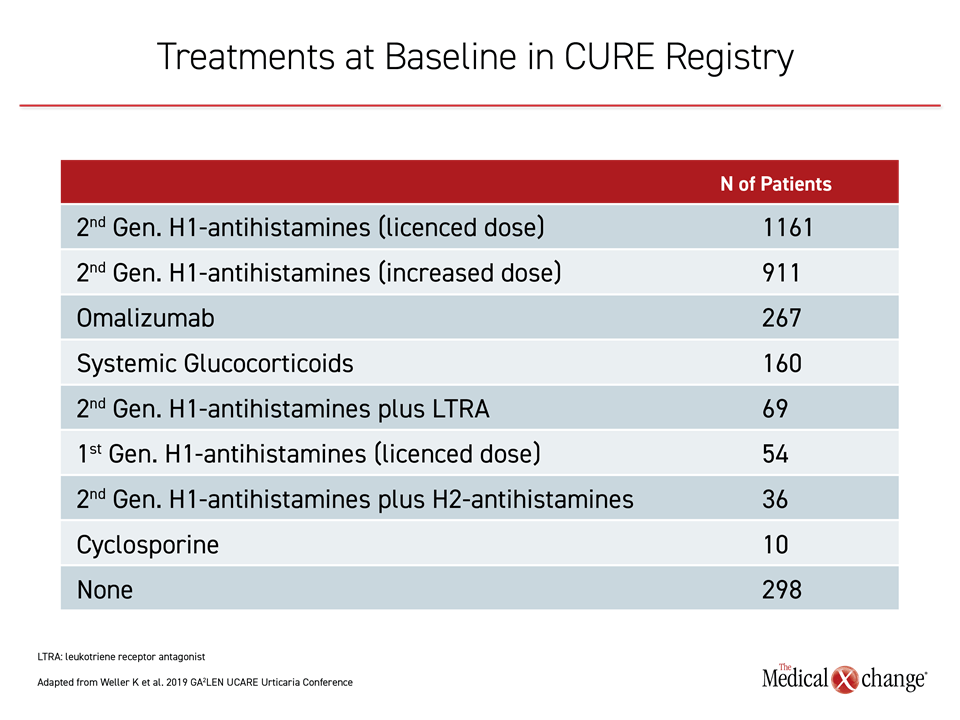
As recommended in the 2018 guidelines, most patients on therapy enrolled in CURE are on a standard dose of second-generation antihistamines, a high dose of second-generation antihistamines, or the monoclonal antibody omalizumab (Table 1). Of those on another therapy, the relatively high proportion on systemic glucocorticoids, which are not recommended for long-term use in the guidelines, was of potential concern. These data informed subsequent discussion of next-step strategies for those who are not adequately controlled on omalizumab with or without continued antihistamine therapy.
Treating Past the Guidelines: The Challenging Patient
On the algorithm recommended in the 2018 guidelines, the majority of patients can expect adequate symptom control, but the options are less clear for the next step in therapy, according to both the 2018 guidelines and the deliberations at the 2019 GA2LEN UCARE Urticaria Conference. For addressing the treatment-refractory patient, the focus of the deliberations was on high doses of omalizumab, the value of cyclosporine, and therapies in development.
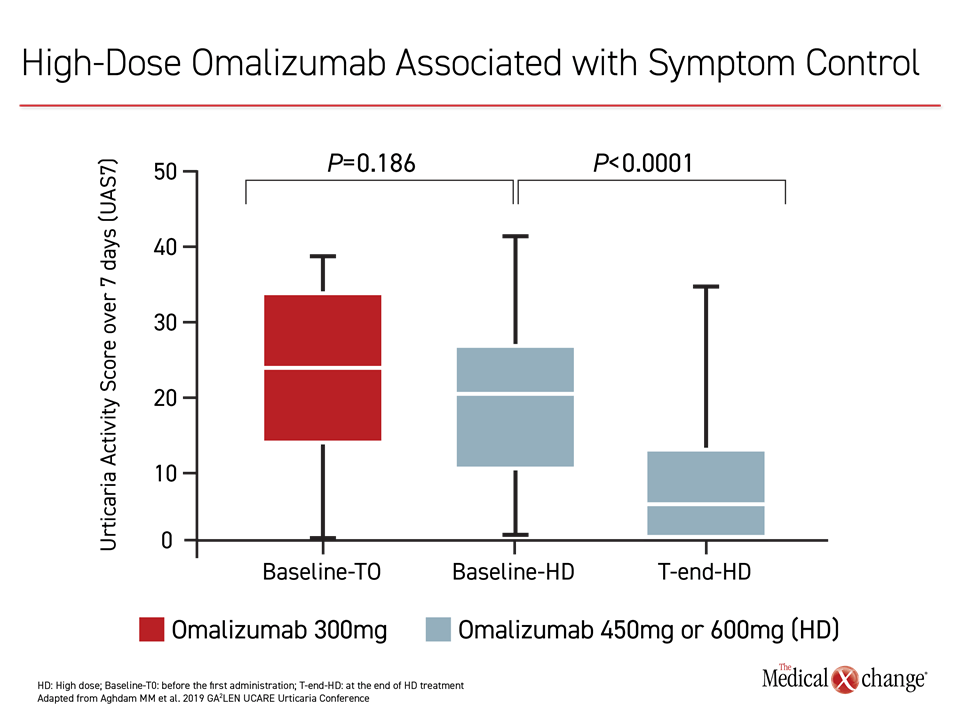
Higher-than-standard omalizumab doses are effective for increasing rates of response, according to several reports and a recently published retrospective analysis updated at the Istanbul meeting. In the recent study with 166 CSU patients, 44 were treated with high-dose omalizumab.2 In this subgroup, the Urticaria Activity Score over 7 days (UAS7) fell for each incremental increase in dose from the standard, which is 300 mg every four weeks (Figure 2).
The greater activity was attributed to greater omalizumab exposure, which can be achieved by increasing the dose on a standard schedule or administering the standard dose more frequently, typically at two-week intervals, according to Dr. Todor A. Popov, Professor, University Hospital Sv. Ivan Rilski, Sofia, Bulgaria. Summarizing the evidence that higher doses of omalizumab offer benefit in those not sufficiently controlled on the standard regimen, he concluded that this is an effective strategy and a reasonable next-step in treatment.
Cyclosporine is a potential alternative. This therapy is effective and “reasonably safe for up to one year,” according to Dr. Allen Kaplan, Division of Pulmonary and Critical Care Medicine, Medical University of South Carolina, Charleston. Showing data that associated cyclosporine with dose-dependent inhibition of histamine release, Dr. Kaplan reported that there is a large literature associating cyclosporine with benefit in CSU. However, he agreed that high-dose omalizumab is a better tolerated and more appropriate option for those not controlled on standard-dose omalizumab. He cautioned, for example, that blood pressure and kidney function in patients on cyclosporine must be monitored every four to six weeks. As a result, he typically substitutes or adds cyclosporine only in those CSU patients who failed to achieve adequate symptom control on high doses of omalizumab, defined as up to 600 mg every two weeks.
Many but not all others agreed with this order of treatment in challenging patients. In an audience poll, 50% selected high-dose omalizumab as the most appropriate next step when standard doses fail. Another 21% selected the addition of cyclosporine to standard-dose omalizumab before testing a high-dose strategy. Twenty-three percent voted for a trial of cyclosporine before high-dose omalizumab. The remainder opted for alternatives, such as dapsone or hydroxychloroquine, before either high-dose omalizumab or cyclosporine, even though the evidence for these alternatives is limited.
For those who selected cyclosporine prior to high-dose omalizumab, concern about cost was an important issue, judging from the discussion that followed the poll. Representatives from countries where formularies are restricted reported that reimbursement for high-dose omalizumab is often difficult to obtain or unavailable, particularly in the context of data that remains limited in refractory patients.
The next set of guidelines is expected to provide more guidance for managing patients who do not respond to the currently recommended first three steps in the algorithm. While acknowledging the need for a guideline-based step after cyclosporine, Dr. Kaplan cautioned that support for the agents often considered as third- or fourth-line options for CSU, such as dapsone, methotrexate, sulfasalazine, and intravenous gamma globulin, is largely confined to case reports. Objective evidence from trials with these or newer agents will be required to alter the current treatment algorithm.
Of agents in development, a newer anti-IgE monoclonal antibody called ligelizumab is widely regarded as the most promising. In a dose-finding phase 2b trial published several weeks prior to the 2019 GA2LEN UCARE conference,3 CSU patients inadequately controlled on high doses of antihistamines were randomized to one of three doses of ligelizumab or the standard 300 mg dose of omalizumab. Each was administered once every four weeks for a period of 20 weeks.
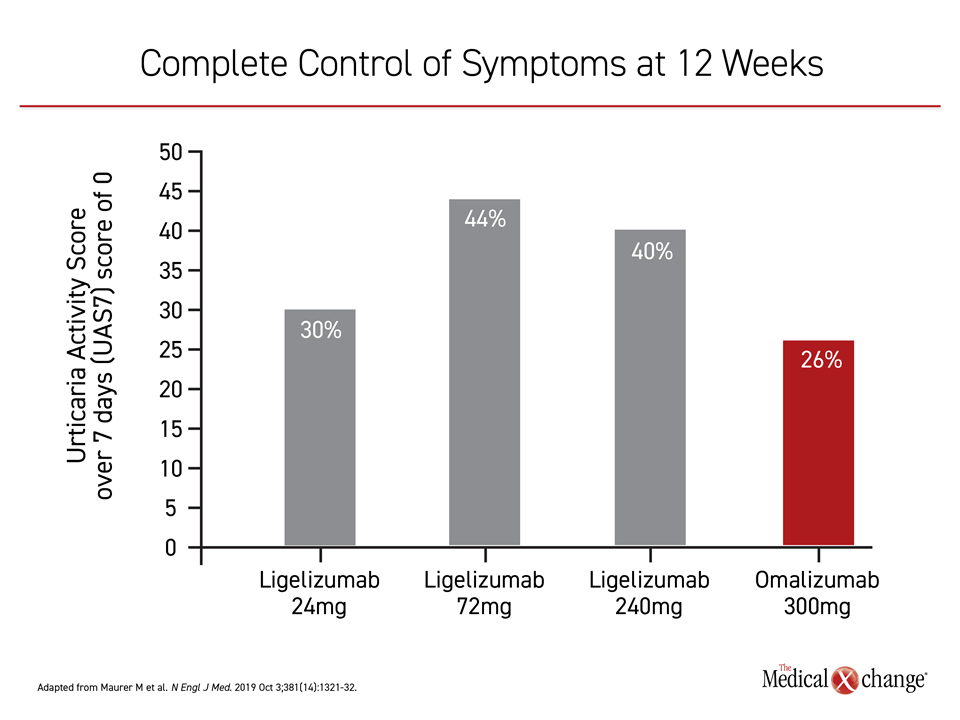
When the four treatment arms were compared, all three doses of ligelizumab achieved a higher rate of complete response, defined as a UAS7 score of 0, than the standard regimen of omalizumab (Figure 3). Injection site reactions occurred more frequently on ligelizumab, but there were no other safety concerns raised with either drug, according to Dr. Ana Gimenez-Arnau, an associate professor of dermatology, Autonomous University, Barcelona, Spain.
“In follow-up of this core study, patients on the highest dose of ligelizumab [240 mg every four weeks] reached a complete response faster [4 vs. 9.5 weeks] than omalizumab,” Dr. Gimenez-Arnau said.
In a subsequent open-label extension of this same phase 2b trial, re-treatment with 240 mg ligelizumab every four weeks was offered to those who redeveloped symptoms during a 24-week washout following the trial. In this extension, which included 226 patients and was presented as an abstract at the 2019 GA2LEN UCARE meeting,4 the rate of adequate response climbed from 58.7% at four weeks to 76.8% at week 20 and to 84.2% at week 52. Response to retreatment did not differ for those randomized to high or low doses of ligelizumab in the controlled trial. A phase 3 trial of ligelizumab is expected.
“We need to understand CSU as a more complex disease than one mediated by IgE alone.”
Encouraged by progress in understanding the molecular events that drive CSU, monoclonal antibodies targeting other immunological mediators are also showing promise, according to Dr. Elias Toubi, Director of the Allergy and Clinical Immunology Department, Bnai Zion Medical Center, Haifa, Israel. He listed several non-specific targets, such as interleukin-17, relevant to other autoimmune diseases as well as novel targets particularly relevant to CSU. One of these novel monoclonal antibodies, known as AK002 (antolimab), targets Siglec-8, a protein found on the surface of mast cells and eosinophils. This drug has reached early stages of clinical testing.
“We need to understand CSU as a more complex disease than one mediated by IgE alone,” Dr. Toubi said. In addition to the downstream events that lead to histamine release, he reported that there is increasing interest in intervening in fundamental processes that drive the autoimmune process, such as deactivation of T cells. He believes a broader set of therapeutic options might also lead to individualization of therapy by urticaria subtype.
Comorbidities: Improving Outcomes in Chronic Urticaria
Neglected in the 2018 guidelines, the burden of comorbidities was a major topic of discussion at the 2019 GA2LEN UCARE meeting. In the absence of a cure for chronic urticaria, effective screening and treatment of comorbidities assume particular importance for the goal of improving quality of life. Currently, there are no widely accepted protocols for managing comorbidities, according to Dr. Mona Al-Ahmad, Department of Allergy, Kuwait Ministry of Health, Al Asimah, Kuwait, but she suggested that the first step is awareness.
“At the initial diagnostic workup, it is important to consider comorbidities, because these are certainly common,” Dr. Al-Ahmad said.
Comorbidities might best be organized within two distinct groups, immunologic and non-immunologic, according to Dr. Al-Ahmad and Dr. Torsten Zuberbier, who joined Dr. Al-Ahmad in evaluating the current evidence. The first author of the joint 2018 EAACI/ GA2LEN/EDF/WAO guidelines, Dr. Zuberbier is affiliated with the Department of Dermatology and Allergy at Charité University of Medicine, Berlin, Germany. He agreed that the management of comorbidities should be integrated into routine care.
“At the initial diagnostic workup, it is important to consider comorbidities, because we have the data to suggest these are common.”
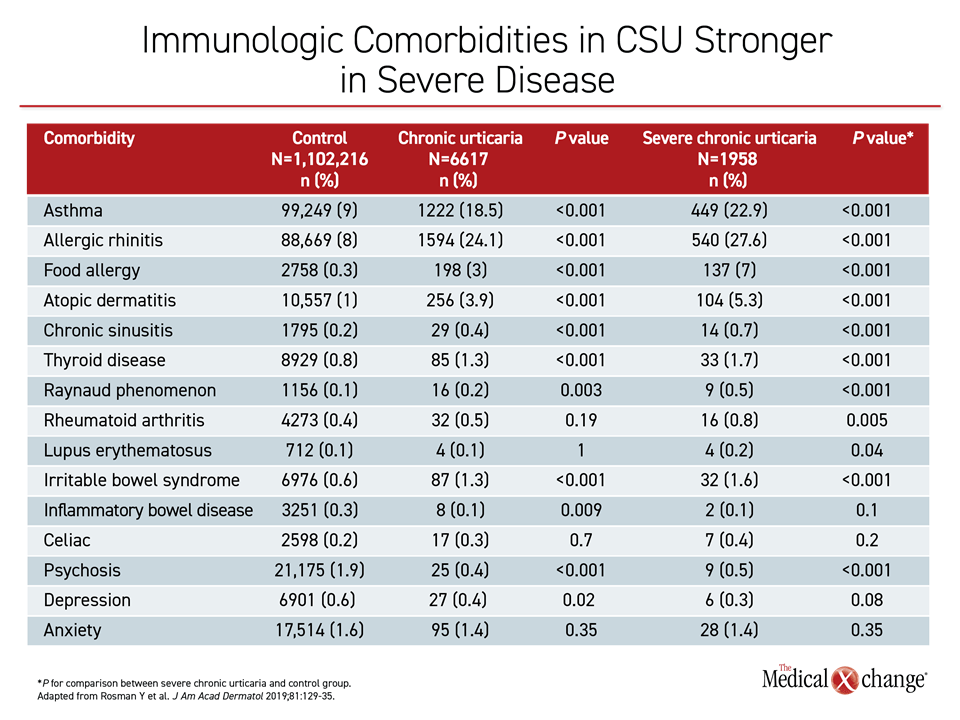
Potentially linked by common pathophysiological pathways, immunologic comorbidities significantly associated with CSU include asthma, Hashimoto’s thyroiditis, Raynaud phenomenon, allergic rhinitis, atopic dermatitis, and diabetes mellitus type 1, according to Dr. Zuberbier. The associations are generally stronger with increased CSU severity (Table 2).
Psychiatric disorders, particularly depression, are among the non-immunologic comorbidities most strongly linked to CSU, but Dr. Al-Ahmad, among others, acknowledged that there continues to be a debate about whether depression is an association or a consequence of chronic CSU symptoms. In either case, Dr. Al-Ahmad suggested that treatment of comorbid depression early in the course of CSU might mitigate its adverse effect on quality of life.
The value of routine screening for comorbidities, whether immunologic or non-immunologic, in CSU remains a topic of debate, but Dr. Zuberbier cited data to support the logical assumption that relief of comorbidities improves CSU outcomes directly or indirectly.5 For example, a small study associated levothyroxine with improvement of urticaria in a subgroup of patients with Hashimoto’s thyroiditis.6 Citing the association between CSU and gastroesophageal reflux disease (GERD),7 Dr. Zuberbier maintained that screening and treating this comorbidity, like others, is potentially warranted for benefits independent of CSU.
Dr. Al-Ahmad made the same point. “We can say that it is important to consider comorbidities when evaluating patients with CSU. Certainly those comorbidities that are identified should be treated, but this is an area for which more data is needed,” she said.
Biomarkers and Disease Monitoring
It is unclear whether biomarkers for the diagnosis, evaluation, and monitoring of CSU will be mature by the time that the next guidelines are written. Intensive efforts to develop biomarkers with the potential to differentiate chronic urticaria subtypes, thereby accelerating time to an effective therapy, are generating promise, but these have not yet led to viable clinical instruments, according to Dr. Simon Francis Thomsen, professor of biomedical sciences, University of Copenhagen, Denmark.
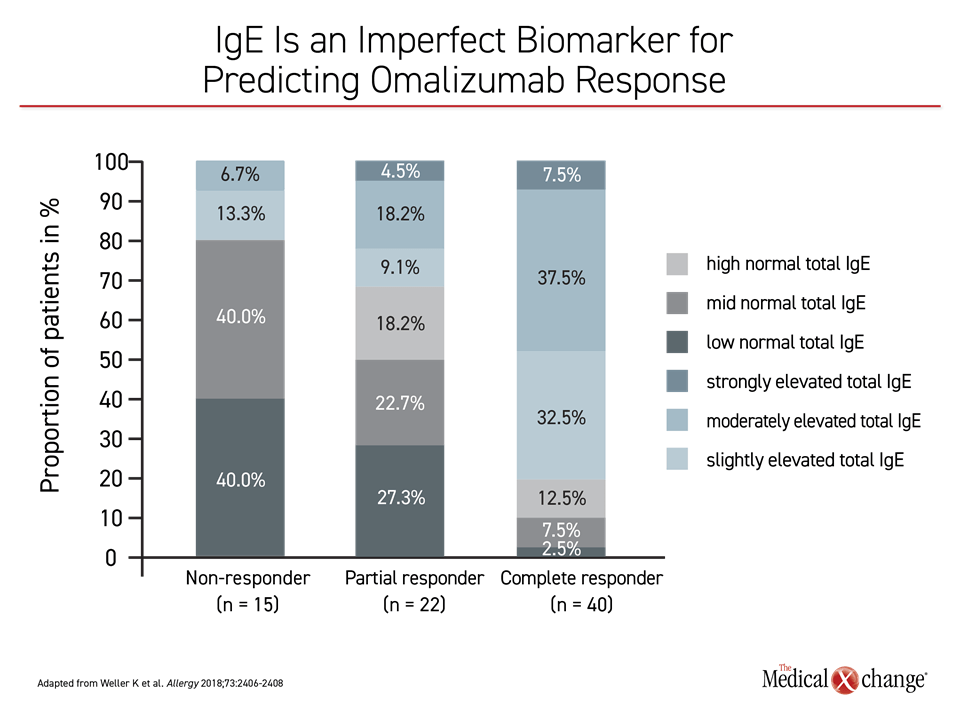
Serum concentrations of D-dimer and IgE were among his examples. Each has shown an association with response to omalizumab, drawing attention for their potential to select patients likely to respond to this agent,8,9 but neither produced a correlation sufficient for clinical application. In the case of IgE, a study at Dr. Weller’s institution showed a relationship between IgE serum concentration and response to omalizumab, but there were responders with low IgE concentrations as well as non-responders with high concentrations10 (Figure 4).
Whether or not clinically useful biomarkers emerge in time for the next set of guidelines, patients remain the best source of information regarding the burden of their disease, according to several experts who addressed this topic. In the 2018 guidelines, several PROs, including UAS7, the Chronic Urticaria Quality of Life questionnaire (CU-QoL), and the Angioedema Activity Score (AAS), were identified as useful tools for monitoring disease and response to therapies. Additional PROs, such as those designed to monitor specific CindUs, such as cold or cholinergic urticaria, were described at the 2019 GA2LEN UCARE meeting. Based on the goal of improving quality of life, it was generally agreed that PROs could be useful for confirming the benefit of treatment strategies.
Summary
Progress in best practices for the management of CSU as well as other forms of chronic urticaria is accelerating as a result of the UCARE collaboration. Initiatives to increase awareness about chronic urticaria and its exceptional burden on quality of life when left untreated are being joined by specific programs to better characterize the disease and advance therapeutic strategies. Global collaborations are already ongoing or will soon be launched to evaluate the impact of diet, physical activities, and other lifestyle factors on urticaria, test the value of combination therapies in urticaria control, characterize CSU in children, and collect data on common CindU subtypes such as cold urticaria and urticarial vasculitis.
In conjunction with real-world urticaria data accrued in CURE, the UCARE collaboration is expected to lead to higher rates of disease control. Practical lessons from the anticipated advances will be included in coming and subsequent treatment guidelines.
References
1. Zuberbier T, Aberer W, Asero R, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy 2018;73:1393-414.
2. Aghdam AM, van den Broek F, Rijken F, Knulst AC. High-dose omalizumab use in patients with chronic spontaneous urticaria. GA2LEN UCARE Urticaria Conference; 2019; Istanbul, Turkey.
3. Maurer M, Gimenez-Arnau AM, Sussman G, et al. Ligelizumab for Chronic Spontaneous Urticaria. N Engl J Med 2019;381:1321-32.
4. Metz M, Baker DR, Chu YC, Danilycheva I, Hua E, Janocha R. Re-treatment with ligelizumab achieves high rates of completely- and well-controlled symptoms in chronic spontaneous urticaria 2019 GA2LEN UCARE Urticaria Conference; 2019; Istanbul, Turkey.
5. Kolkhir P, Borzova E, Grattan C, Asero R, Pogorelov D, Maurer M. Autoimmune comorbidity in chronic spontaneous urticaria: A systematic review. Autoimmun Rev 2017;16:1196-208.
6. Kim DH, Sung NH, Lee AY. Effect of Levothyroxine Treatment on Clinical Symptoms in Hypothyroid Patients with Chronic Urticaria and Thyroid Autoimmunity. Ann Dermatol 2016;28:199-204.
7. Aitella E, De Bartolomeis F, Savoia A, Fabiani M, Romano M, Astarita C. The overlap syndrome of urticaria and gastroesophageal reflux disease. PLoS One 2018;13:e0207602.
8. Asero R, Marzano AV, Ferrucci S, Cugno M. D-Dimer Plasma Levels Parallel the Clinical Response to Omalizumab in Patients with Severe Chronic Spontaneous Urticaria. Int Arch Allergy Immunol 2017;172:40-4.
9. Puxeddu I, Petrelli F, Angelotti F, Croia C, Migliorini P. Biomarkers In Chronic Spontaneous Urticaria: Current Targets And Clinical Implications. J Asthma Allergy 2019;12:285-95.
10. Weller K, Ohanyan T, Hawro T, et al. Total IgE levels are linked to the response of chronic spontaneous urticaria patients to omalizumab. Allergy 2018;73:2406-8.