Infectious Disease
2020 Conference on Retroviruses and Opportunistic Infections (CROI)
HIV Patients with NAFLD Are at High Risk of Progressive Liver Fibrosis
Boston – An uncommon opportunity to evaluate the natural history of non-alcoholic fatty liver disease (NAFLD) in patients with HIV has demonstrated that fibrosis progression is accelerated in these patients relative to the general population. Furthermore, the study, presented at the 2020 CROI meeting, supports the premise that visceral fat is a treatable predictor of advancing liver disease in the HIV population.
These data, generated from a subanalysis of a randomized trial, are important because it is already known that NAFLD progresses in patients with HIV even if the infection is well controlled. There is an urgent need for new strategies to control NAFLD, which is now predicted to replace hepatitis C infection as the most common cause of liver-related death in the HIV population.
Benefits with Fat Reductions
“Visceral adiposity was found to be a novel clinical predictor of accelerated hepatic disease, which suggests that therapies to reduce visceral fat may be particularly effective in NAFLD associated with HIV,” reported Dr. Lindsay T. Fourman, Metabolism Unit, Massachusetts General Hospital, Harvard Medical School, Boston. She presented data newly derived from a randomized trial that was published a few months prior to the CROI meeting (Stanley TL et al. Lancet HIV 2019;6:e821-e830).
“Therapies to reduce visceral fat may be particularly effective in NAFLD associated with HIV.”
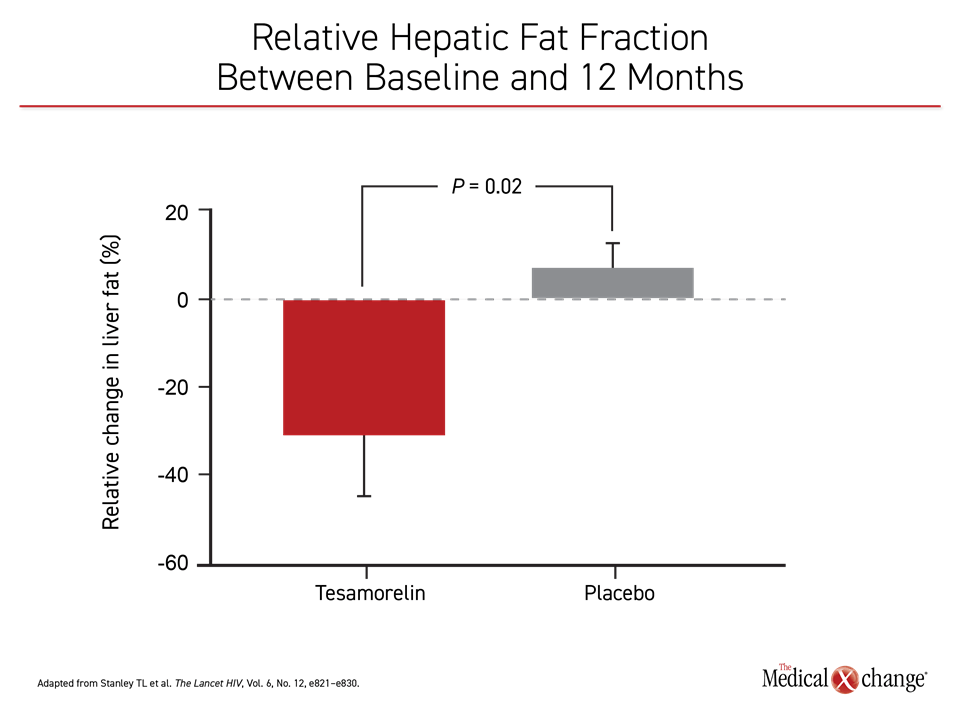
In the published trial, the growth hormone-releasing hormone (GHRH) tesamorelin was compared to placebo in HIV-infected patients with NAFLD. At 12 months, tesamorelin was associated with a large reduction in liver fat content as measured with the hepatic fat fraction (HFF) relative to those randomized to placebo (Figure 1).
In this new analysis, the natural history of HIV-associated NAFLD was explored in the placebo group by comparing baseline and end-of-study liver biopsies. It is already known that fibrosis is the strongest predictor of both liver-specific mortality as well as all-cause mortality in NAFLD patients, but the placebo population in this randomized trial provided an opportunity to look specifically for baseline predictors of fibrosis development and progression in an HIV-positive population.
Placebo Population Is Representative
When the 24 patients in the placebo arm were compared to the overall sample of 58 patients, mean duration of HIV infection (18 vs. 16 years) body mass index (BMI) (32 vs. 31), waist circumference (113 vs. 110 cm), visceral fat (247 vs. 243 cm2) and subcutaneous fat (324 vs. 300 cm2) were comparable. In both groups, 33% had non-alcoholic steatohepatitis (NASH) at baseline. The NAFLD activity scores (NAS) were 2.8 and 2.7 in the placebo and overall population samples, respectively.
Similar to those randomized to the active treatment arm, 43% of those randomized to placebo had fibrosis at baseline. While stage 4 fibrosis at baseline was a study exclusion, stages 1 (36%), 2 (40%), and 3 (24%) fibrosis were represented.
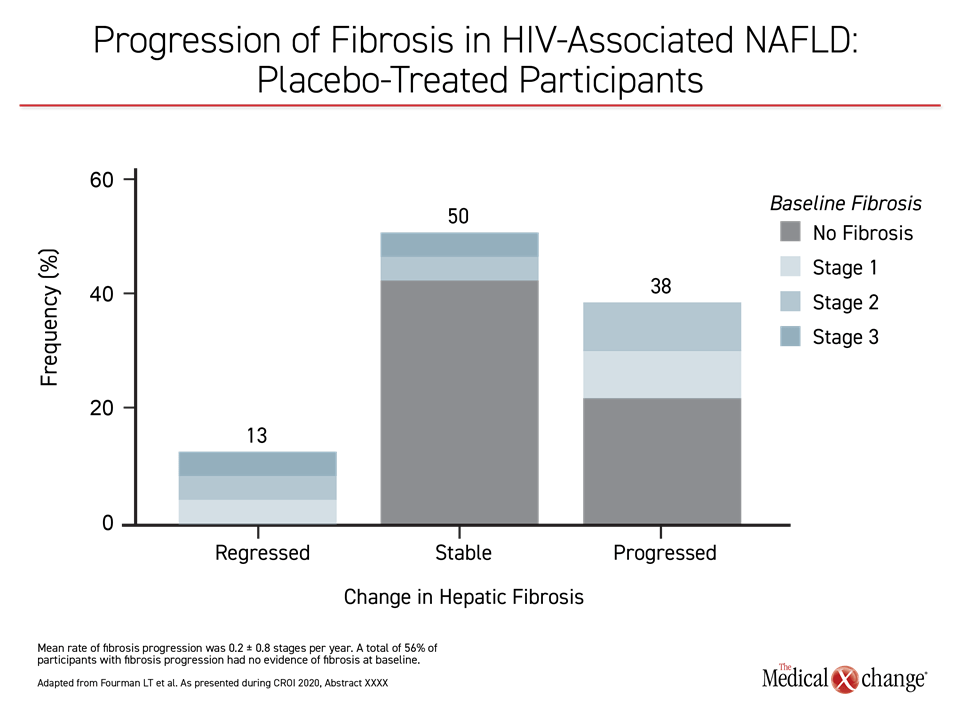
At the end of 12 months, 13% of those with baseline fibrosis regressed and 38% progressed. While the remaining patients remained stable, 56% of those who did have fibrosis progression did not have detectable fibrosis at baseline. For the total population, the mean rate of fibrosis progression was 0.2 stages/year but reached rates as high 0.8 stages/year.
Visceral Fat Predicts Fibrosis Progression
In a multivariate analysis of potential baseline predictors of fibrosis progression, visceral fat emerged as a significant predictor, increasing the odds ratio (OR) by 37% (OR 1.37, P=0.03). Other characteristics tested, including baseline NAS score, BMI, and liver fat, did not show significant predictive value on either univariate or multivariate analysis. Over the course of the study, fibrosis progression was significantly associated with increases in C-reactive protein (P=0.02), HbA1c (P=0.02), and NAS score (P<0.0001).
In this “first longitudinal assessment of a well-defined sample with HIV-associated NAFLD, we were able to leverage liver biopsy specimens to demonstrate that there is a high prevalence and progression of liver fibrosis,” Dr. Fourman said (Figure 2). Compared to an expected fibrosis progression rate of 0.03 stages/year that has been reported in NAFLD patients without HIV, the rate of 0.2 stages/year in this study appears to confirm a far accelerated risk of end-stage liver disease even among patients, like those in this study, who were virally suppressed on contemporary antiretroviral therapy.
“We were able to leverage liver biopsy specimens to demonstrate that there is a high prevalence and progression of liver fibrosis in the HIV population with NAFLD.”
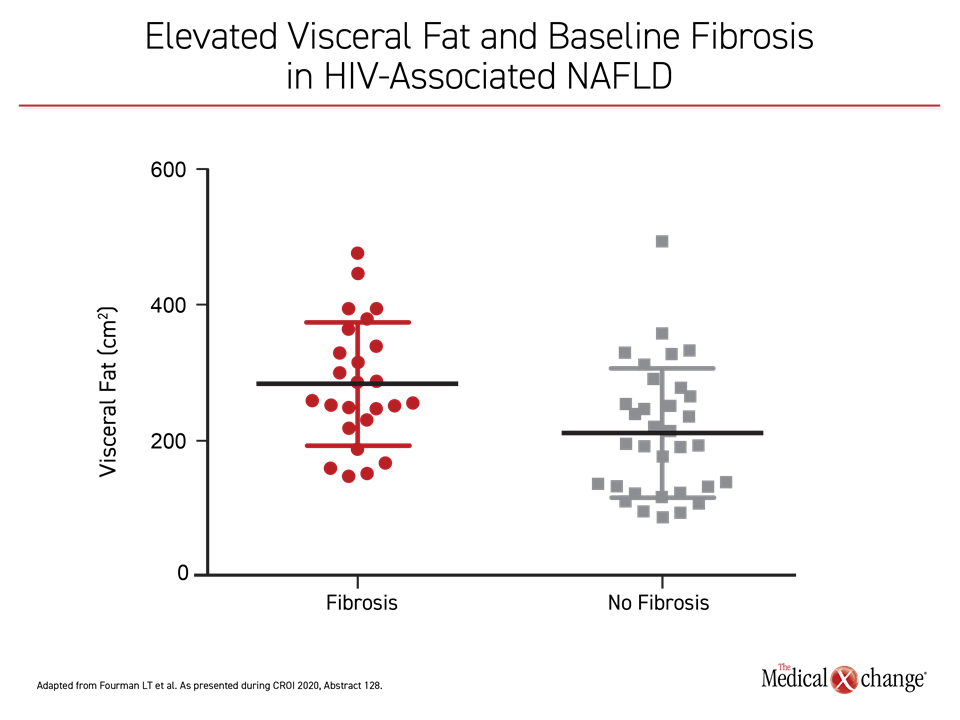
Fibrosis severity is a strong predictor of both liver-specific and all-cause mortality in patients with or without HIV, but the risk is particularly acute in patients with HIV, where the estimated prevalence is at least 25%, according to Dr. Fourman. In this study, elevated visceral fat was a significant predictor (P=0.005) of the presence of fibrosis at baseline (Figure 3). Currently, there are no pharmacologic therapies for NAFLD.
“The fibrosis progression rate of 0.2 stages/year appears to confirm a far accelerated risk of end-stage liver disease even when HIV is well controlled.”
GHRH Analogue Reduced Liver Fat
In the randomized trial from which this new analysis was derived, the GHRH analogue tesamorelin demonstrated promise for this indication. The primary endpoint was change in HFF, a tool for quantifying NAFLD. At 12 months, there was a modest rise from baseline in HFF in the placebo arm, but a nearly 35% HFF reduction in those on tesamorelin (P=0.02). When compared for the proportion of patients who achieved HFF <5%, the difference was even greater between the tesamorelin and placebo groups, respectively (35% vs. 4%; P=0.0069).
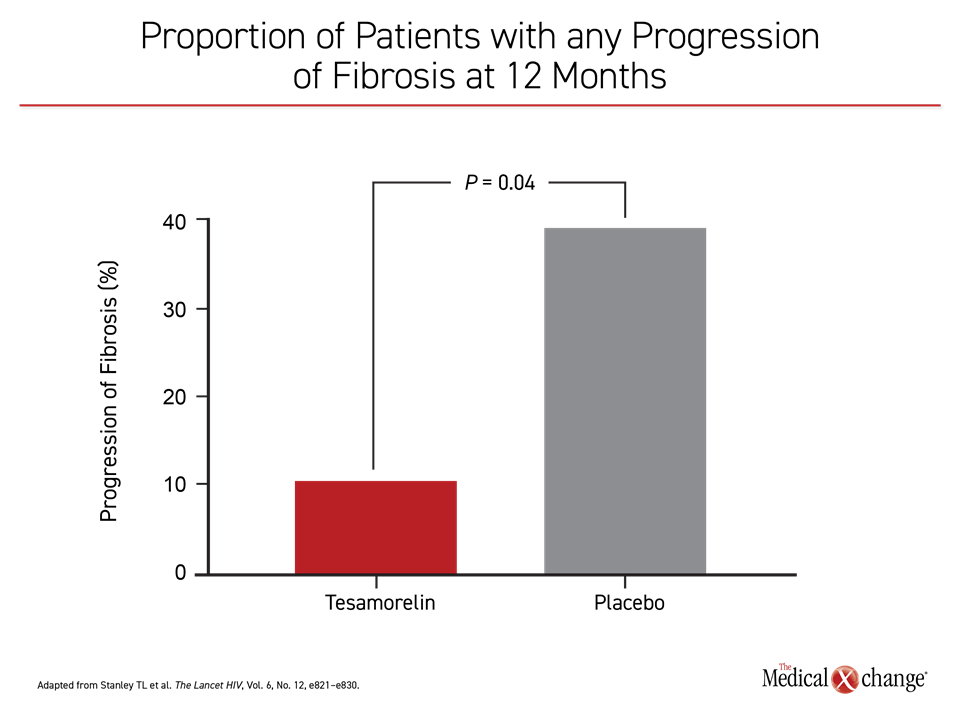
In addition, the reduction in HFF was accompanied by a large relative reduction in the proportion of patients with any progression of fibrosis at 12 months (Figure 4). Tesamorelin received regulatory approval almost 10 years ago for the treatment of excess abdominal fat in HIV patients with lipodystrophy. These data suggest that this drug, which was well tolerated in this and other controlled trials, might have a role to play in preventing end stage liver disease in HIV patients with NAFLD.
Conclusion
Patients with HIV face a high risk of NAFLD and an accelerated rate of fibrosis progression compared to those without HIV even when HIV is well controlled. Reductions in liver fat with the GHRH analogue tesamorelin are associated with attenuation of liver fat as well as progressive fibrosis in HIV patients, suggesting promising treatment options toward a potential threat for liver-related mortality.