Cardiology
2020 American College of Cardiology (ACC) Virtual Scientific Sessions
Major Trials Alter Standards for Anticoagulant Therapy in PAD and Valve Replacement
Virtual Meeting – Two multinational trials are poised to alter standards of care in areas of antithrombotic therapy. Presented as latebreakers during the virtual ACC meeting, one trial, called VOYAGER PAD, associated a direct oral anticoagulant (DOAC) with improved outcomes after revascularization for peripheral artery disease (PAD). The other, called POPular TAVI, found oral anticoagulation alone to be a superior strategy to oral anticoagulation plus an antiplatelet agent after transcutaneous aortic valve replacement (TAVI). Both studies were published at the time of their presentation in the New England Journal of Medicine.
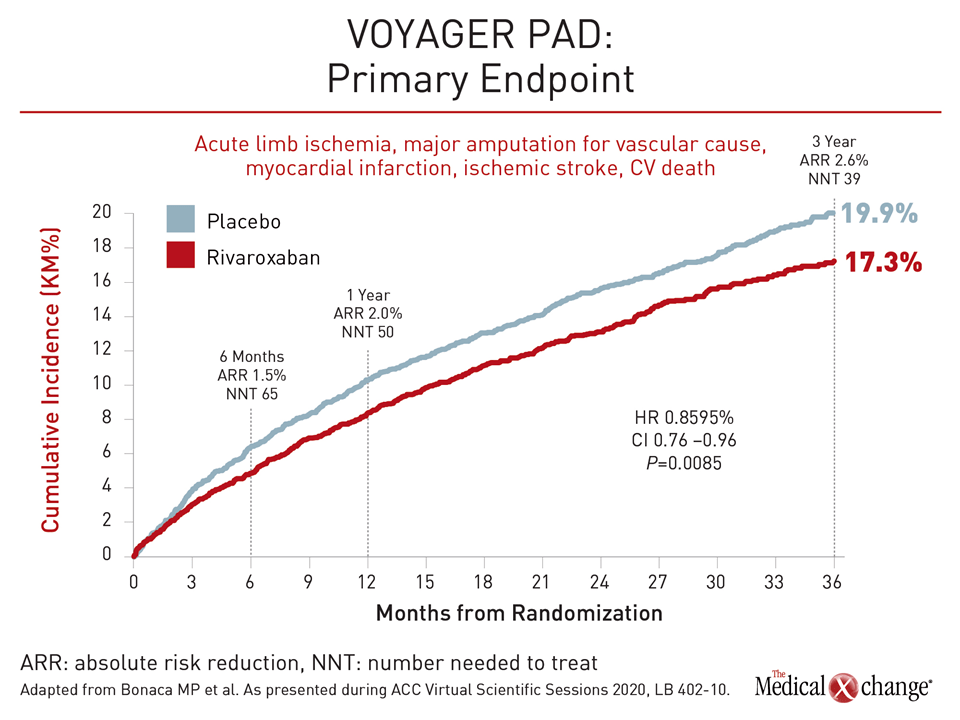
The VOYAGER PAD trial randomized 6564 symptomatic PAD patients to 2.5 mg twice daily of the DOAC rivaroxaban or placebo following a successful revascularization procedure. Patients in both groups were taking low-dose acetylsalicylic acid (ASA). For the primary composite endpoint of acute limb ischemia, major amputation for vascular causes, myocardial infarction (MI), ischemic stroke, or death from cardiovascular (CV) causes, dual pathway inhibition with rivaroxaban plus ASA resulted in a 15% relative risk reduction (P=0.009) at the end of three years (Figure 1).
“The benefits of rivaroxaban over ASA alone were apparent early and continued over time and were consistent across major subgroups,” reported Dr. Marc P. Bonaca, Director of Vascular Research, University of Colorado School of Medicine, Aurora. “Rivaroxaban was associated with an increase in ISTH [International Society on Thrombosis and Haemostasis] bleeding but no excess in intracranial or fatal bleeding.”
“The benefits of rivaroxaban over ASA alone were apparent early and continued over time and were consistent across major subgroups.”
Major Knowledge Gap Addressed
Prior to VOYAGER PAD, there had been no class 1 evidence-based recommendations for antithrombotic therapy following revascularization in PAD patients, according to Dr. Bonaca. Due to the abundant evidence that PAD patients are at high risk of vascular events following revascularization, he believes VOYAGER PAD has addressed a major knowledge gap. On top of the already high risk for major adverse CV events (MACE) for any patient with documented PAD, those who require revascularization face a greatly increased risk of events following the procedure, Dr. Bonaca said.
“After revascularization for symptomatic PAD, one in five have acute limb ischemia, major amputation for a vascular etiology, MI, ischemic stroke or death at three years,” Dr. Bonaca said. This rate of complications, documented previously, was reflected in the ASA-only arm of the VOYAGER PAD trial.
“After revascularization for symptomatic PAD, one in five have acute limb ischemia, major amputation for a vascular etiology, MI, ischemic stroke or death at three years.”
In the double-blind VOYAGER PAD study, which involved centers in 34 countries including Canada, patients were randomized a median of five days after revascularization. Of the revascularization procedures, 35% were surgical and the remaining were performed with an endovascular or hybrid procedure. At entry, patients were required to be at least 50 years of age and free of risk factors for increased bleeding.
Half of Patients Also on an Antiplatelet Agent
The trial population had multiple high-risk features, including diabetes in 40%, established coronary artery disease in 32% and impaired renal function in 20%. Thirty-five percent of those randomized were current smokers. Nearly all of the participants had a history of claudication prior to revascularization, and approximately 35% had undergone revascularization for PAD previously. At enrollment, slightly more than half of patients were taking the antiplatelet agent clopidogrel, which was permitted by investigator discretion for up to six months following revascularization.
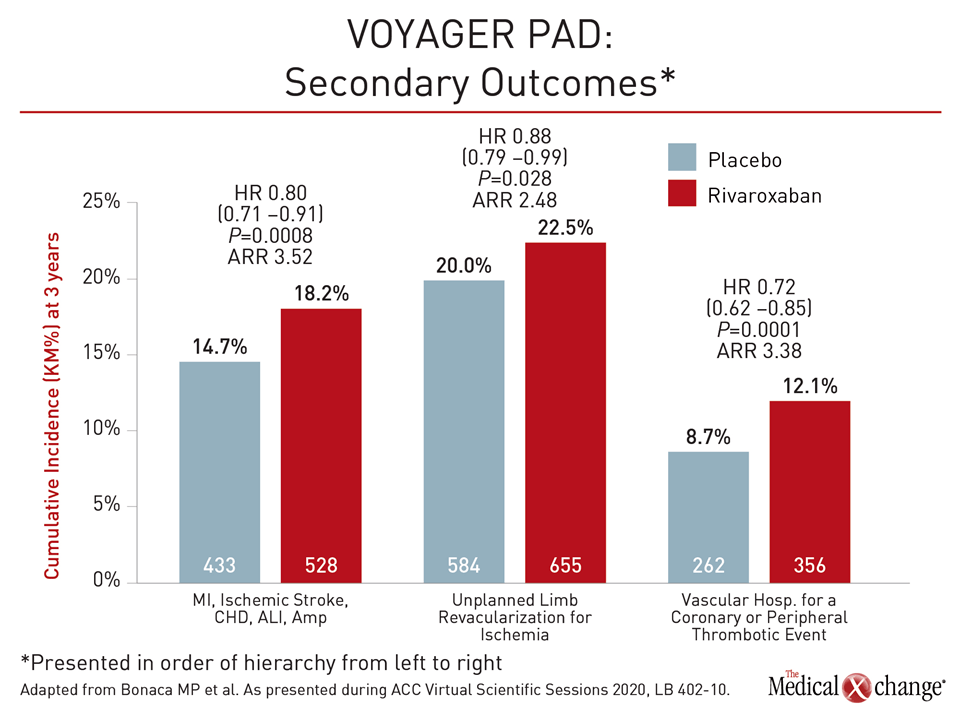
When the components of the primary outcome were evaluated separately, all but CV death occurred at a numerically lower rate in the rivaroxaban arm relative to the placebo arm. When individual components were calculated there was a 33% reduction (5.2% vs. 7.8%) in the risk of acute limb ischemia (95% CI; 0.55-0.82). Unplanned limb revascularization was significantly less common (20% vs. 22.5%) in the arm randomized to rivaroxaban plus ASA (95% CI 0.79 – 0.99; P=0.03). (Figure 2).
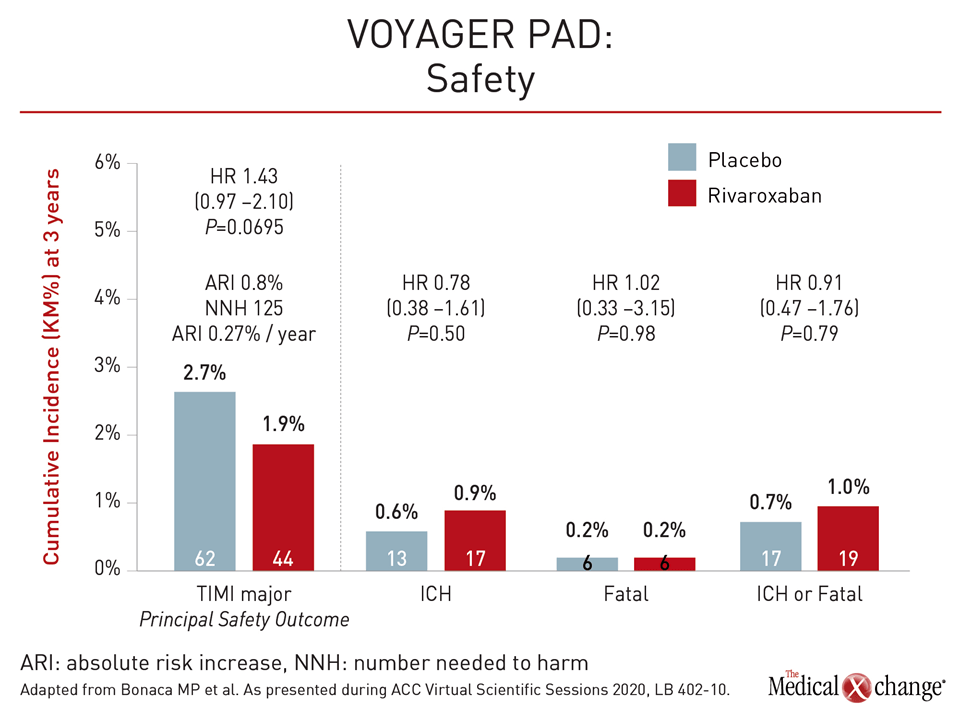
The safety profile was reassuring, according to Dr. Bonaca. Although ISTH bleeding was significantly increased and the greater rate of TIMI major bleeding approached significance in the rivaroxaban arm (2.7% vs. 1.9%; P=0.0695), the combined rate of intracranial hemorrhage or fatal bleeds, which are the most feared bleeding events, was numerically lower among those receiving rivaroxaban (0.7% vs. 1.0%; P=0.79) (Figure 3).
VOYAGER PAD Builds on COMPASS Data
The VOYAGER PAD trial builds on the previously published COMPASS trial. In COMPASS, published more than two years ago (Eikelboom et al. N Engl J Med 2017;377:1319-1330), 27,395 patients with stable atherosclerotic vascular disease (ASVD) were randomized to 2.5 mg rivaroxaban twice daily plus low-dose ASA, 5 mg rivaroxaban twice daily, or low-dose ASA alone. Although the twice-daily 5 mg rivaroxaban dose did not significantly reduce the primary composite outcome of CV death, stroke, and MI relative to ASA alone, those receiving the 2.5 mg twice daily dose of rivaroxaban plus ASA had a 24% reduction in the hazard ratio (HR) for these events (HR 0.76; 95% CI, 0.66 – 0.86; P<0.001).
The risk of ISTH major bleeding was significantly increased (P<0.001) with the 2.5 mg dose of rivaroxaban in the COMPASS trial, but the risk of intracranial hemorrhage and fatal bleeds, although numerically higher, was not. In a calculation of net clinical benefit that included both the reduction in vascular events and the increase in fatal or critical organ bleeding events, rivaroxaban was associated with an overall 20% reduction in relative risk of an adverse outcome (HR 0.80; P<0.001).
High-risk patients, including those enrolled in COMPASS with PAD, did best. In the subgroup of 7470 patients with PAD, the net clinical benefit climbed to 25% (HR 0.75; P=0.011), according to a subsequently published analysis (Anand et al. Lancet 2018;391:219-229). This was attributed to a greater relative reduction in the risk of the primary composite COMPASS outcome (HR 0.72; P=0.0047) with no greater risk of bleeding than observed in the full study population.
Net Benefit Greater in High-risk Groups
The greater relative risk reduction also extended to patients who entered COMPASS with diabetes, according to a new substudy presented at the ACC 2020 virtual meeting. Although the relative risk reduction in the primary efficacy endpoint for those randomized to 2.5 mg rivaroxaban relative to ASA alone was only slightly greater in those with diabetes (HR 0.78; P=0.02) relative to those without (HR 0.81; P=0.01), the absolute benefits in the efficacy endpoints were larger in those patients with diabetes.
“Use of dual pathway inhibition with low-dose rivaroxaban plus ASA is particularly attractive in high-risk patients, such as those with diabetes,” reported Dr. Deepak L. Bhatt, Executive Director of Interventional Cardiovascular Programs, Brigham and Women’s Hospital, Boston. The COMPASS diabetes substudy results were simultaneously published in Circulation.
“Use of dual pathway inhibition with low-dose rivaroxaban plus ASA is particularly attractive in high-risk patients, such as those with diabetes.”
Another COMPASS substudy, published two years ago, anticipated the clinical benefit of a reduction in acute limb ischemia observed in VOYAGER PAD. In that substudy (Anand et al. J Am Coll Cardiol 2018;71:2306-2315), incident limb ischemia was strongly associated with adverse outcomes, including several fold increases in subsequent hospitalization (HR 7.21; P<0.0001), subsequent amputations (HR 197.5; P<0.0001), and death (HR 3.23; P<0.001).
Acute Limb Ischemia is Therapeutic Target
Based on these results, Dr. Sonia Anand, Professor of Cardiology, McMaster University, Hamilton, Ontario, called prevention of major adverse limb events “of utmost importance” at the time that the COMPASS subanalysis was published.
In the previously published COMPASS PAD substudy, for which she also served as first author, Dr. Anand speculated, “Low-dose rivaroxaban twice a day with ASA could replace ASA alone as a standard of care in stable PAD patients who are not at high risk of bleeding.” The VOYAGER PAD data validate this conclusion in PAD patients who have undergone revascularization.
In VOYAGER PAD, “we found that adding low-dose rivaroxaban after revascularization for PAD significantly reduced the spectrum of complications that we fear most in PAD, which is acute limb ischemia, major amputation, heart attack, and stroke. This benefit was not only seen early on but was maintained over time,” Dr. Bonaca concluded.
POPular TAVI Trial Is Practice Changing
The POPular TAVI trial, another ACC 2020 latebreaker devoted to anticoagulation after a CV intervention, was also considered practice changing. In this non-inferiority trial, 313 patients with a prior indication for anticoagulation who were undergoing TAVI were randomized to remain on anticoagulation alone or receive clopidogrel in addition to their anticoagulation for three months. Two primary composite outcomes were evaluated over a period of 12 months. The first was all bleeding and the second included non-procedural bleeding.
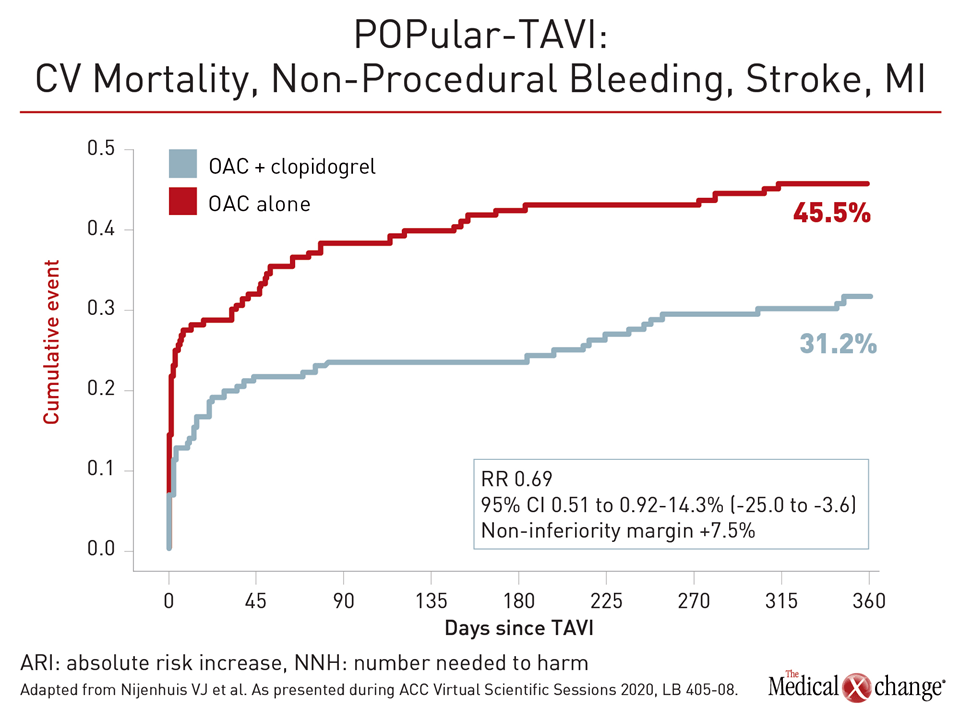
There were two secondary composite outcomes. The first was for CV mortality, non-procedural bleeding, stroke, and MI. For this outcome, oral anticoagulation alone was associated with a lower risk of events at 12 months, although the P value for this non-inferiority endpoint was not calculated (Figure 4). For the other composite secondary outcome of CV mortality, stroke, and MI, events, absolute rates were lower in the anticoagulation only arm (13.4% vs. 17.3%), but this relative risk (RR) reduction of 23% was not significant (RR 0.77; 95% CI 0.46-1.31). However, the difference between arms did not approach the prespecified non-inferiority margin of 7.5%.
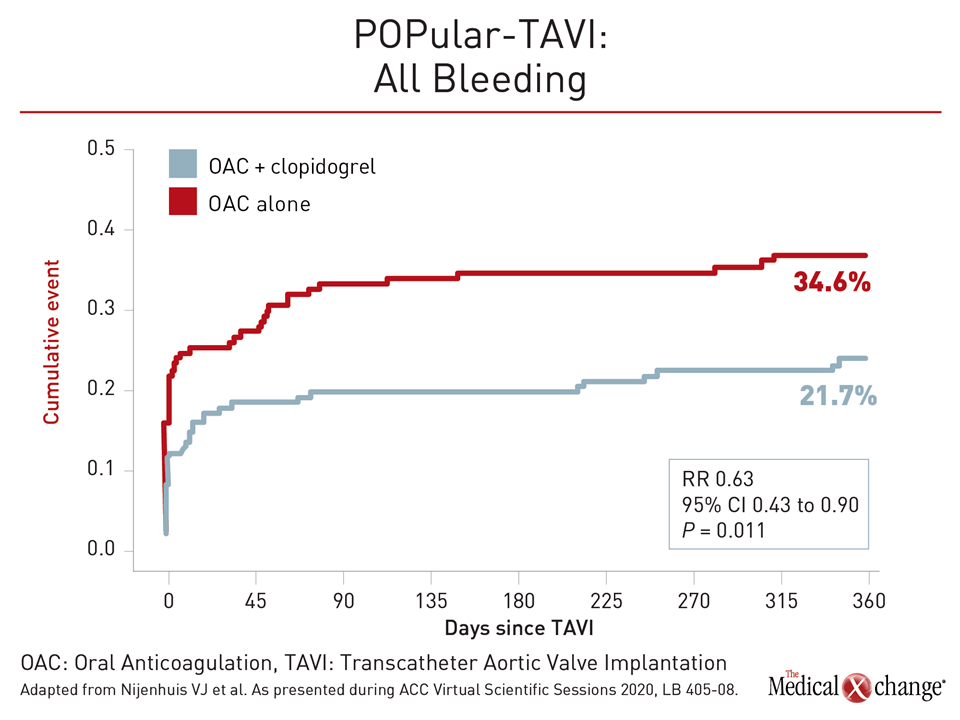
Importantly, and consistent with the major hypothesis of the trial, the 34% relative risk reduction in the rate of bleeding in the anticoagulation arm (21.4% vs. 34.6%) did reach statistical significance (RR 0.63; P=0.011) (Figure 5).
POPular TAVI Addresses Knowledge Gaps
“This study helps physicians better understand the risks of adding antiplatelet therapy to oral anticoagulants in TAVI patients,” reported the senior author, Dr. Vincent J. Nijenhuis, a cardiologist affiliated with St. Antonius Hospital, Nieuwegein, the Netherlands.
“This study helps physicians better understand the risks of adding antiplatelet therapy to oral anticoagulants in TAVI patients.”
Separate data for POPular TAVI, which involved 17 participating centers in Europe, were collected on candidates for TAVI who did not have a prior indication for anticoagulation, but these results were not presented at the ACC. All TAVI patients were eligible for the study except for those who had received a drug-eluting stent in the previous three months or a bare metal stent in the previous month. The type of oral anticoagulation offered was left to the discretion of the investigator. Approximately 75% received a vitamin K antagonist such as warfarin. The remaining 25% received a DOAC of which apixaban and rivaroxaban were used most commonly.
The current guidelines for antithrombotic therapy following TAVI are based on expert opinion in the absence of well-controlled trials, according to Dr. Nijenhuis. Most guidelines endorse a combination of anticoagulation and antiplatelet therapies. The POPular TAVI trial now provides guidance for the subpopulation of TAVI patients with a prior indication for oral anticoagulants.
In these patients, anticoagulation alone relative to anticoagulation plus clopidogrel “reduces the risk of major bleeding events, including life-threatening or disabling bleeding but does not increase the rate of thrombotic events,” Dr. Nijenhuis concluded. He indicated that these data define a new standard for antithrombotic therapy in this patient population.
Conclusion
Two latebreaker trials have provided a new evidence basis for optimally balancing antithrombotic and bleeding risks following common interventions. For patients with PAD undergoing revascularization, low doses of the DOAC rivaroxaban plus ASA were associated with a reduction in vascular events. Despite an increase in bleeding events, there was no excess risk observed in life-threatening bleeding. For patients with a prior indication for oral anticoagulation undergoing TAVI, the addition of the antiplatelet agent clopidogrel increased risk of bleeding without significantly lowering vascular risks.