Hematology
25th European Hematology Association Congress (EHA25 Virtual)
Next-Generation BTK Inhibitors Fulfilling Promise for CLL in Long-Term Follow-Up
Virtual Meeting – New follow-up data presented at EHA25 Virtual have greatly expanded the evidence that next-generation Bruton tyrosine kinase (BTK) inhibitors are providing far more durable control in the B-cell malignancies to which they have been compared than the first-generation agent. In chronic lymphocytic leukemia (CLL), follow-up data showing durable benefit with a next-generation BTK inhibitor is now out to four years, confirming sustained disease control with no unexpected toxicity.
The new data support the premise that the greater BTK selectivity of the next-generation therapies provides efficacy and safety advantages relative to ibrutinib, the first BTK inhibitor. This included follow-up data out to a median of 55 months with acalabrutinib, which was recently approved in Canada for first-line and relapsed/refractory (R/R) CLL. Other next-generation BTK inhibitors in development, such as zanubrutinib, are also showing high rates of activity and low relative levels of toxicity in B-cell diseases.
90% Improvement in Progression-Free Survival at 28 Months
The data from the ELEVATE-TN and ASCEND trials, which were conducted in treatment-naïve and R/R CLL, respectively, provided the basis for the recent acalabrutinib approval in Canada. Both studies associated this next-generation BTK inhibitor with a favourable safety and efficacy profile. Now, new follow-up data show the effect is sustained.
“Even as a monotherapy in the long-term follow-up, acalabrutinib achieved high and persistent responses regardless of genomic features,” reported Dr. John C. Byrd, chairman of leukemia research, Ohio State University Comprehensive Cancer Center, Columbus.
“Even as a monotherapy in the long-term follow-up, acalabrutinib achieved high and persistent responses.”
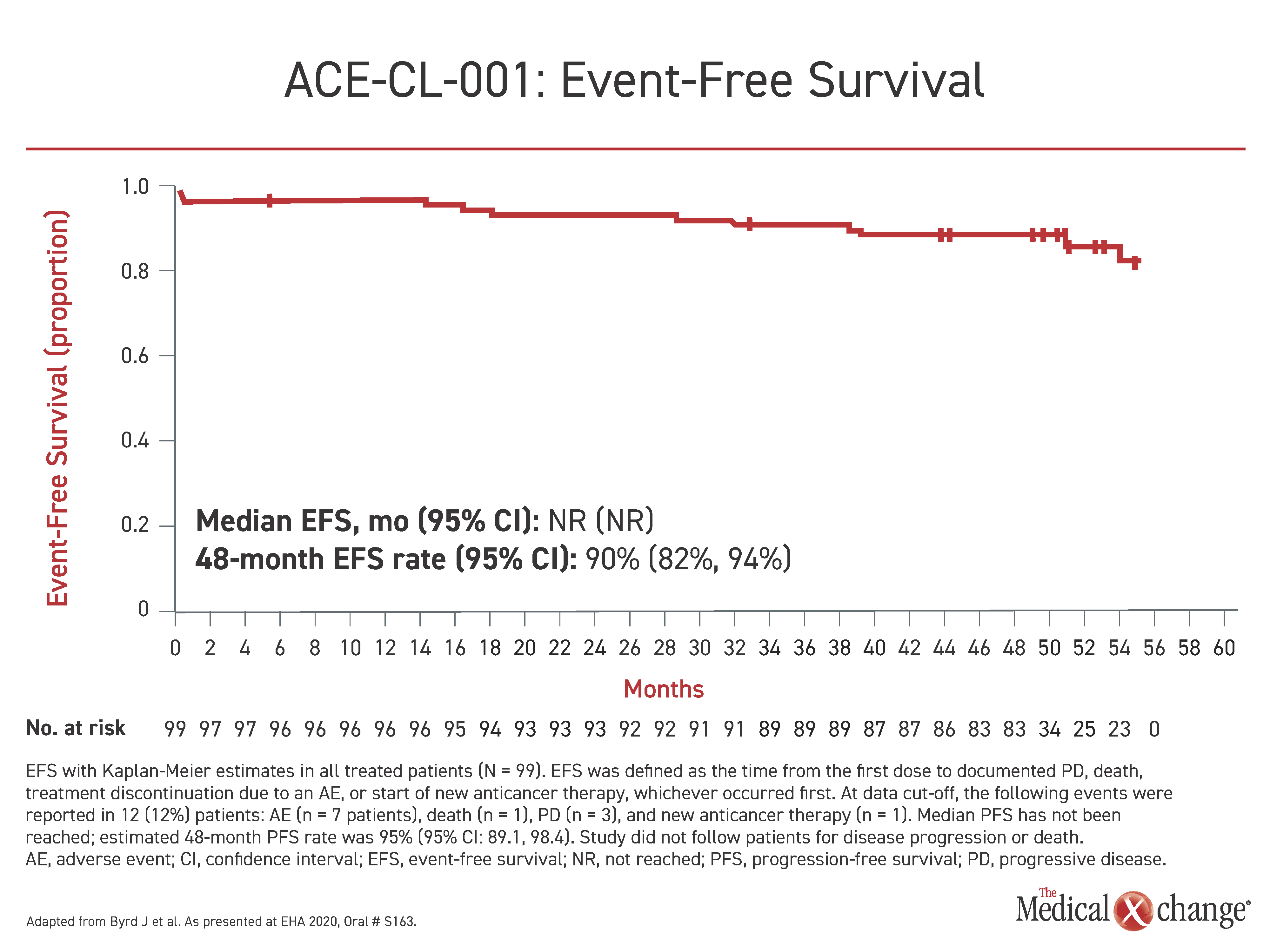
The long-term follow-up data were derived from ACE-CL-001, the first phase 2 trial with acalabrutinib in treatment-naïve patients. With a 97% overall response rate (ORR), the initial response rates were reported to be similar across groups defined as high-risk by RAI stage, presence of bulky disease, or adverse genomic features. Now, after a median of more than four years (53 months) of follow-up, disease control is being sustained in most of those initially enrolled. At the 48-month timepoint, the median duration of response (DOR), event-free survival (EFS), and progression-free survival (PFS) have not been reached, Dr. Byrd reported. The proportion of patients in EFS at 48 months is 90% (Figure 1). The PFS at 48 months was 96%.
Of the 99 patients who entered the trial, 85 (86%) are still receiving acalabrutinib. Only three patients have left the study due to progressive disease. Only six discontinued due to an adverse event.
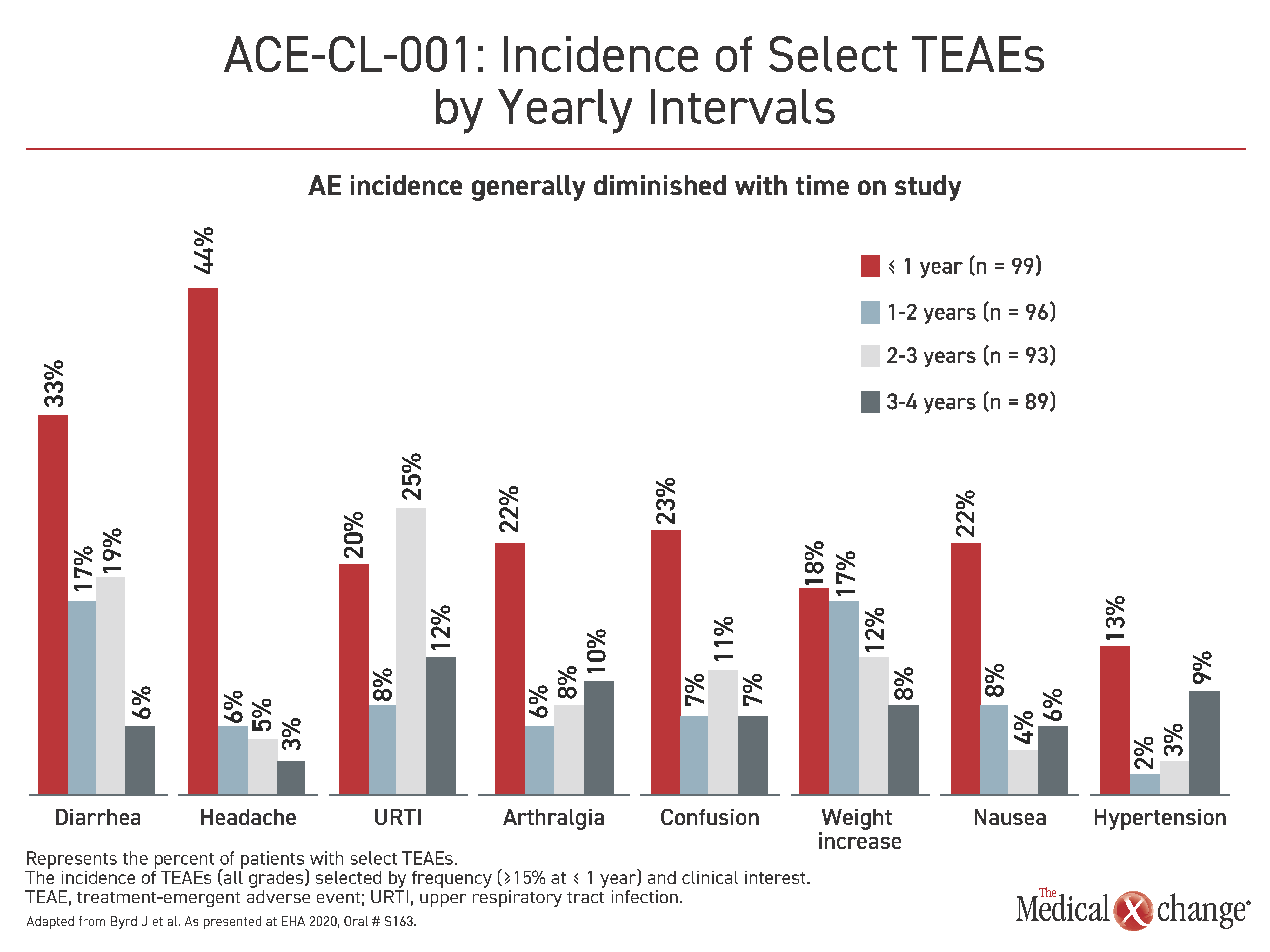
There have been no new adverse events observed over the long-term follow-up, but Dr. Byrd did report that all of the most common adverse events, including diarrhea, headache, nausea, and hypertension diminished over the period of extended treatment (Figure 2).
With these data, which represent “the longest safety and efficacy follow-up to date with acalabrutinib in treatment-naïve CLL patients,” Dr. Byrd said that evidence confirms that sustained benefit follows the high rates of response previously reported.
ASCEND Final Results
Final results from the phase 3 ASCEND trial, which established the efficacy of acalabrutinib in R/R CLL were also presented at EHA25 Virtual. In that trial, 310 patients were randomized to 100-mg twice-daily acalabrutinib or investigator’s choice of rituximab plus idelalisib (IdR) or bendamustine.
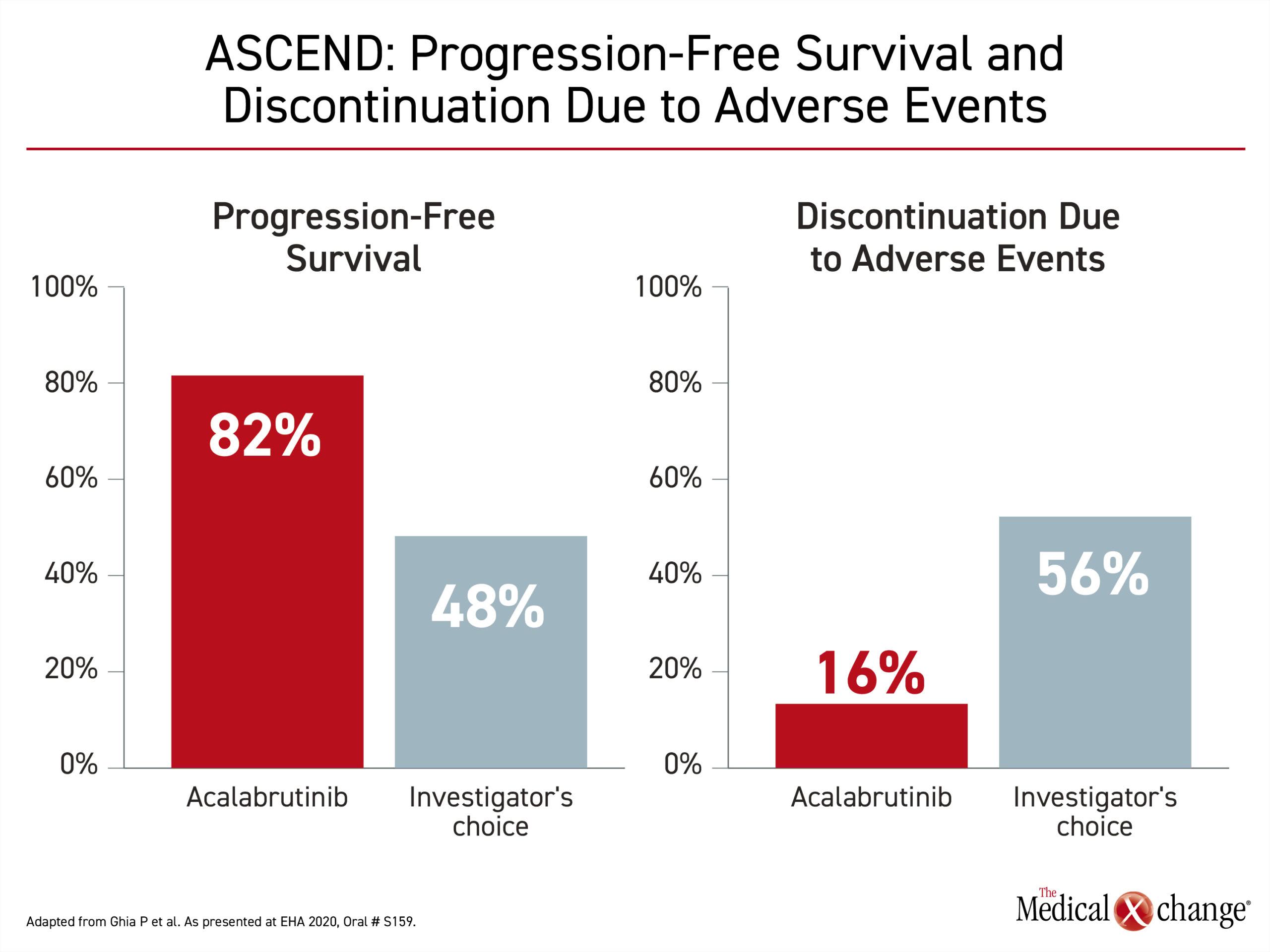
“At a median follow-up of 22 months, the median PFS was not reached in the acalabrutinib arm,” reported Dr. Paolo Ghia, Director of the Strategic Research Program on CLL, University Vita-Salute San Raffaele, Milan, Italy. Relative to a median PFS of 16.8 months, the rates of PFS at 18 months were 82% and 48% for the acalabrutinib and comparator arms respectively, producing a 77% risk reduction (HR 0.23; P<0.0001).
“At a median follow-up of 22 months, the median PFS was not reached in the acalabrutinib arm.”
At 80% in both arms, there is no difference in overall survival (OS) rate in follow-up so far, but acalabrutinib was far better tolerated. The rates of study discontinuations due to an adverse effect were 16% versus 56% (Figure 3).
Data for Waldenström’s Macroglobulinemia
The promise of other next-generation BTK inhibitors, such as zanubrutinib, is also attributed to relative BTK selectivity. In Waldenström’s macroglobulinemia (WM), the next-generation zanubrutinib showed greater activity than ibrutinib in the head-to-head phase 3 ASPEN trial even though significance for superiority on the primary combined endpoint of complete (CR) and very good partial responses (VGPR) was missed (28.4% vs 19.2%; P=0.092).
“Other indicators, such as investigator-assessment of clinical response [30.4% vs. 18.2%; P=0.03] did suggest an advantage for zanubrutinib,” reported Dr. Meletios Dimopoulos, Chairman, Department of Clinical Therapeutics, University of Athens, Greece.
Fewer treatment discontinuations for adverse events in the zanubrutinib arm (4.0% vs. 9.2%) support the premise that the greater selectivity of the newer BTK inhibitors will confer an advantage for safety and efficacy relative to first-generation ibrutinib.
Conclusion
Long-term data support exceptional activity for the next-generation BTK inhibitors in CLL and potentially other B-cell malignancies. By demonstrating relatively low rates of serious toxicities and durable response, the trial data validate the importance of BTK specificity.