Neurology
Multiple Sclerosis: Expert Review and Commentary from Published Literature
Relapsing-Remitting Multiple Sclerosis and High-efficacy Therapies
Anthony Traboulsee, MD
Director, MS Clinic and Clinical Trials Research Group
Centre for Brain Health
University of British Columbia
Vancouver, British Columbia
For patients with relapsing-remitting multiple sclerosis (RRMS) uncontrolled on frontline treatments, there are now numerous options that improve disease control measured by annualized relapse rate (ARR). Only a subset of these therapies reverses functional deterioration measured by Expanded Disability Status Scale (EDSS). Drugs that reduce ARR relative to first-generation therapies are labeled high-efficacy agents, but protection against neurodegeneration represents a more rigorous measure of benefit. High-efficacy agents such as fingolimod stabilize RRMS on the basis of ARR. Immunosuppressants such as alemtuzumab reverse functional decline. Like other potent agents used for induction in RRMS, the therapeutic window for alemtuzumab is relatively narrow, encouraging selective application for those informed of treatment goals and risks.
Road to Current Treatment Options
In 1993, interferon beta-1b became the first therapy licensed to improve the natural history of RRMS.1 It was soon joined by interferon beta-1a and glatiramer acetate, two other injectable drugs associated with modest reductions in relapse relative to placebo. These traditional standards for initial treatment of RRMS have an extensive and favorable safety record but their protection against disease progression has been marginal.2 Over the past decade, the growing array of additional disease-modifying therapies (DMT) for RRMS has allowed treatment to be individualized. High-efficacy therapies initially reserved for patients inadequately controlled on first-generation agents have fostered evolving strategies for appropriate drug sequencing not only to reduce symptomatic episodes of RRMS but to delay progression.
For a 3-part video series with Dr. Anthony Traboulsee focused on the management of RRMS and on the impact to clinical practice, click here
There are many ways to categorize the available therapies for RRMS, including by mode of delivery, mechanism of action, efficacy, and safety. The first-generation therapies were self-administered by injection. Several oral therapies have become available since the first oral agent, fingolimod, was licensed almost 10 years ago. The first therapy administered by intravenous infusion, mitoxantrone, has been available for almost 20 years, but it has now been joined by several targeted monoclonal antibodies, which, in addition to alemtuzumab, include natalizumab and ocrelizumab.
The mechanisms of action differ among available therapies. While the first-generation agents are considered to be immunomodulators, the most effective agents eliminate or at least limit the function of immune cells that drive the autoimmune pathology in the central nervous system (CNS). Alemtuzumab, like ocrelizumab, cladribine, and mitoxantrone, are characterized as immune cell depleting,3 whereas natalizumab is an anti-trafficking agent that inhibits immune cells from participating in the inflammatory CNS attack.4
Treatment Selection
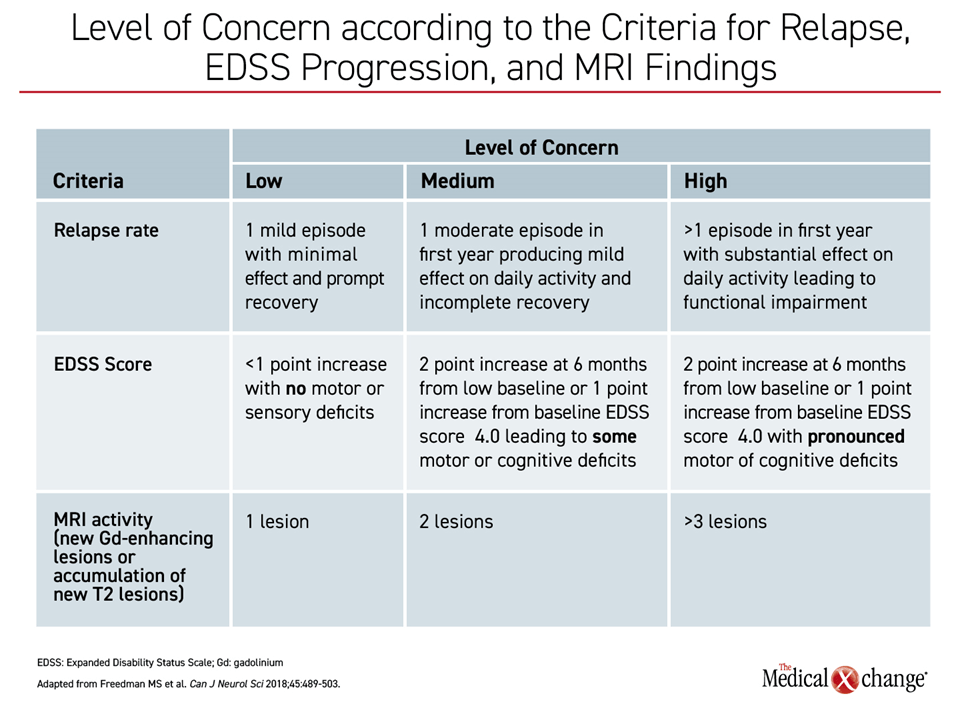
Due to this array of therapy options, several competing treatment algorithms have emerged. Until recently, the escalation paradigm was predominant. Based on a safety-first emphasis, patients have been typically initiated on a first-generation therapy or other less intensive drugs and switched only when control was considered inadequate.5 Although the definition of inadequate control has been controversial, an approach proposed by the Canadian MS Working Group in 2013 encouraged escalation on the basis of uncontrolled disease activity in the form of symptoms or MRI activity.6 In a more recent review, specific criteria were described for low, medium, and high level of concern for progression (Table 1).
However, the failure of a reduction in the rate of relapse to correlate with protection from disability has raised concern, challenging the concept of escalation based on symptoms. In a review of 22 meta-analyses, evidence of long-term benefit on standard DMT therapy was inconsistent despite strong evidence of a short-term inhibition of relapse episodes.7 In the extension trials of first-generation agents, substantial rates of disability were accompanied by rising rates of conversion to secondary progressive multiple sclerosis (SPMS) when follow-up extends beyond five to seven years.8 Due to the concern that patients administered agents with modest efficacy are not receiving optimal protection against disease progression, earlier use of highly-active agents has been proposed, particularly for those with early signs of aggressive disease.9
The principle of moving more effective therapies forward in the treatment of RRMS is attractive, but it is not straightforward. Due to the fact that predictors of aggressive disease are not well established and more effective therapies are generally accompanied with greater risk of adverse events, patient preferences are important for guiding treatment strategy. For patients who have participated in a careful and comprehensive review of potential risks and benefits, many might opt for a therapy likely to prevent disability than one well tolerated but with an unclear ability to prevent disease progression.
Protecting the Brain: The Evolution
Mitoxantrone and natalizumab were early examples of therapies associated with reversal of disability. In a placebo-controlled trial that evaluated mitoxantrone on an every-three-month schedule, significant improvements in EDSS were observed at the end of two years.10 In the pivotal AFFIRM trial with natalizumab, there was a 69% increase in the cumulative probability of improvement in EDSS score at the end of two years relative to placebo in a post hoc analysis.11
However, significant adverse events have limited the utility of both agents. For mitoxantrone, dose-related cardiotoxicity suggests lifetime exposure should not exceed 140 mg/m2.12 Natalizumab was initially withdrawn from the market based on its association with progressive multifocal leukoencephalopathy (PML), a life-threatening complication. Due to a degree of efficacy that had not been observed with other DMTs at the time of its withdrawal, it was subsequently reintroduced for clinical use with caution about managing PML risk.13 Although the threat of PML is reduced through risk-mitigation programs,14 natalizumab is commonly reserved for inadequate response to first- or second-line options.
In the phase 3 CARE-MS II study with alemtuzumab, ARR and sustained accumulation of disability were co-primary endpoints.15 The study enrolled RRMS patients who had relapsed on first-line therapy. The comparator was interferon beta-1a. Patients received alemtuzumab once daily for five days at baseline with a second three-day course administered at 12 months. The interferon was administered three days per week for the duration of the study.
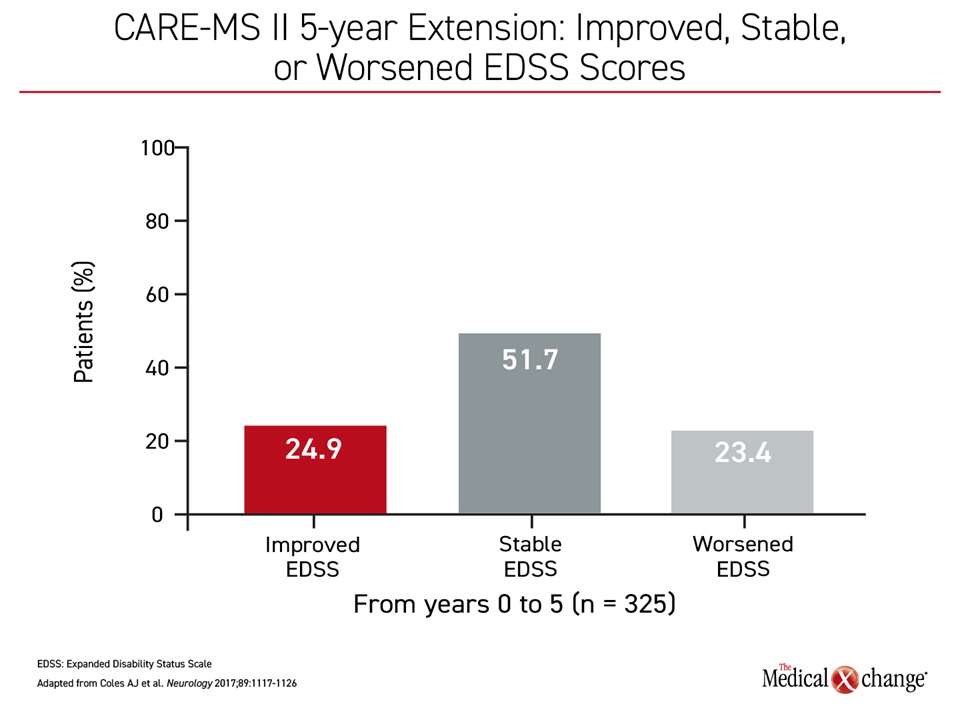
At the end of two years, the large relative advantage for alemtuzumab for both co-primary endpoints was highly significant. For example, there was a greater than 40% reduction in the hazard ratio (HR) for sustained disability at the end of two years (HR 0.58; P=0.008). However, the extension studies showed even greater protection against disease progression. In a three-year extension, which included 92% of those who participated in the randomized trial, 24.9% of the patients who received alemtuzumab had an improvement in EDSS from baseline.16 Most of the remaining had stable EDSS score over the extended follow-up (Figure 1).
Cumulatively, 51.8% of patients were free of clinical disease activity in years three to five of the extension, and 48.6% showed no MRI lesion activity. These outcomes were reinforced by improvements from baseline in several other functional measures, such as the Multiple Sclerosis Functional Composite (MSFC) score. Approximately half of patients randomized to alemtuzumab had improvement in all seven of the EDSS functional domains.17
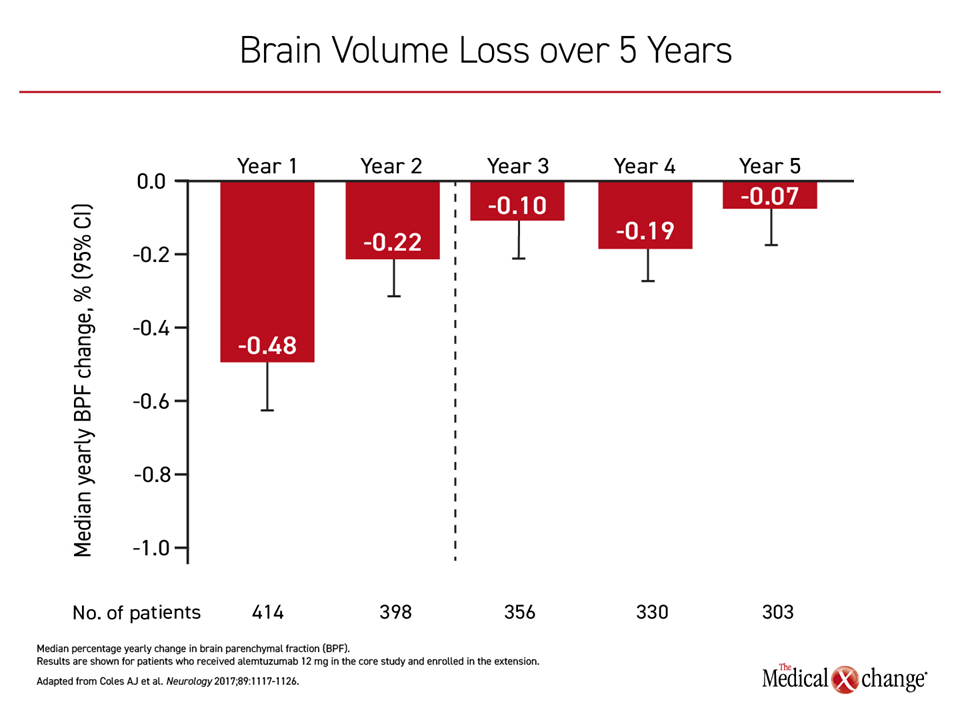
Importantly, and potentially relevant to an ability of alemtuzumab to prevent disease progression, brain volume loss was reduced over time in a second extended follow-up study called TOPAZ18 (Figure 2). No other MS therapy has had a similar magnitude of impact on preventing ongoing brain atrophy despite a similar impact on reducing ARR. In this ongoing follow-up of patients who completed the four-year CARE-MS II extension study and entered into an additional five-year extension, the mean EDSS score improved by 0.17 points, and 70% of patients had stable or improved EDSS score through a median of eight years of follow-up.19
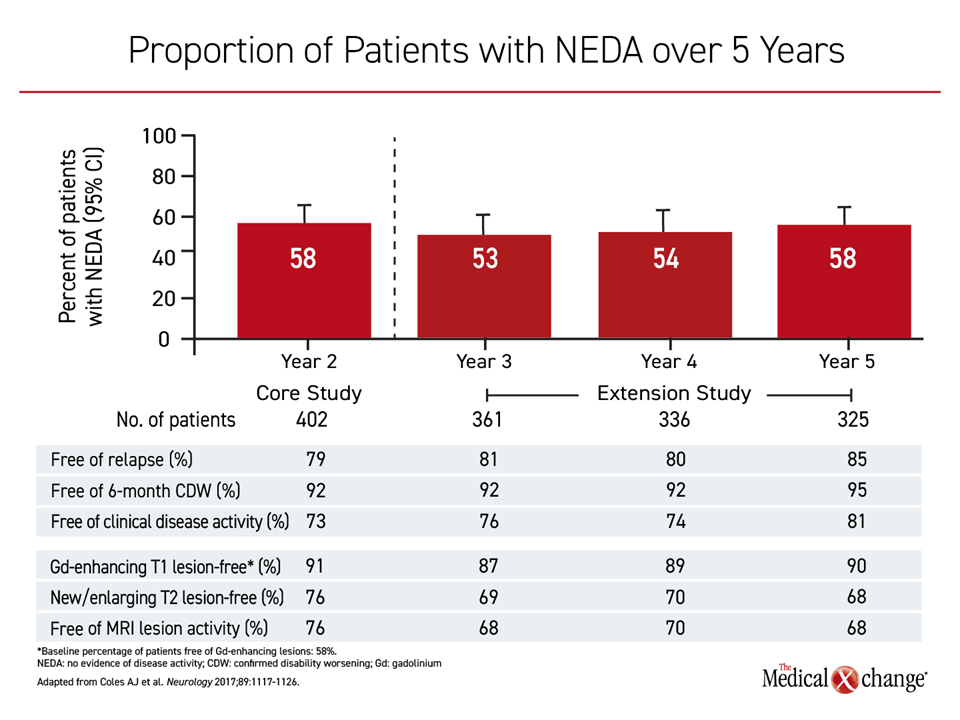
These benefits were achieved even among patients who received no or little additional therapy after the phase 3 protocol. During the extension period, when alemtuzumab was offered as needed, only 20.4% received an additional course of the agent in year three of the extension. Rates were even lower in years 4 and 5. Other DMTs were offered, but fewer than 10% of the patients took an RRMS therapy other than alemtuzumab over this period. Yet, the proportion of patients with no evidence of disease activity (NEDA) remained unchanged at 5 years of follow-up relative to 2 years (Figure 3).
It is uncertain whether other widely-used immune cell-depleting agents provide similar benefits. The pivotal phase 3 trial with cladribine, an oral agent, was placebo-controlled and employed ARR as the primary endpoint.20 The two phase 3 trials with ocrelizumab, an anti-CD20 monoclonal antibody, also employed ARR as the primary endpoint, although interferon beta-1a was the comparator.21 Each of the trials associated the experimental agent with an advantage over the comparator for ARR and relative protection against sustained progression of disability. There was a 33% relative increase in the proportion of patients with a sustained improvement in EDSS compared to interferon.
Induction Regimens: Treating Pathology
Escalation strategies were initially considered to be an appropriate response to an expanding array of therapies for RRMS. Relative to no therapy, first-generation immunomodulators reduce risk of relapse and are well tolerated. Interest in alternative strategies, such as induction regimens, is driven by the goal of more profoundly altering the disease course. As opposed to immunomodulators, therapies that deplete immune cells, such as mitoxantrone and alemtuzumab, target factors that participate in the autoimmune process.
Induction strategies, whether used upfront or after other treatments have failed, are not a recent concept.22 In a long-term observational study published more than 10 years ago, induction mitoxantrone followed by a maintenance immunomodulator was associated with sustained disease control for at least five years.23 In a study that evaluated maintenance glatiramer acetate after cyclophosphamide induction, significant reductions in ARR and gadolinium-enhancing lesions were observed at the end of two years.24
Alemtuzumab is the most broadly studied agent for RRMS induction treatment. Following treatment with alemtuzumab, CD4+ T and B cells can be depleted for up to one year. This has been associated with a reprogramming of the immune system.25 When immune cells are repopulated over time, the proportion of proinflammatory cell types is reduced— an effect credited with restoring immune tolerance networks. Timing might be important. Alemtuzumab has not shown protection against SPMS, suggesting that treatment with alemtuzumab should precede extensive CNS damage.22
Earlier and more frequent use of induction therapies is based on an evolution in treatment goals. Instead of modifying the activity of immune function, which so far has had limited ability to prevent progression to disability, therapies that reduce inflammatory activity are targeted at altering the disease process, preventing structural damage and preserving brain function.26 Although patients with aggressive RRMS are typically considered the more appropriate candidates for this approach, disease course is difficult to predict. Based on the definition of disability within five years of diagnosis, aggressive MS is only encountered in about 10% of patients,27 but almost all RRMS patients face eventual progression. In a study of RRMS patients followed for 15 years, only 2.9% had EDSS <3 and no evidence of cognitive impairment.28
Patient Selection: Assessing Benefit to Risk Ratio
Therapies with potent effects on immune function commonly impose risks, requiring a benefit-to-risk calculation. Alemtuzumab is not an exception. Although infusion-associated reactions are the most frequently reported adverse events in clinical studies, it is important to inform patients about the risk of infections and secondary autoimmune diseases, which are risks increased on alemtuzumab treatment.
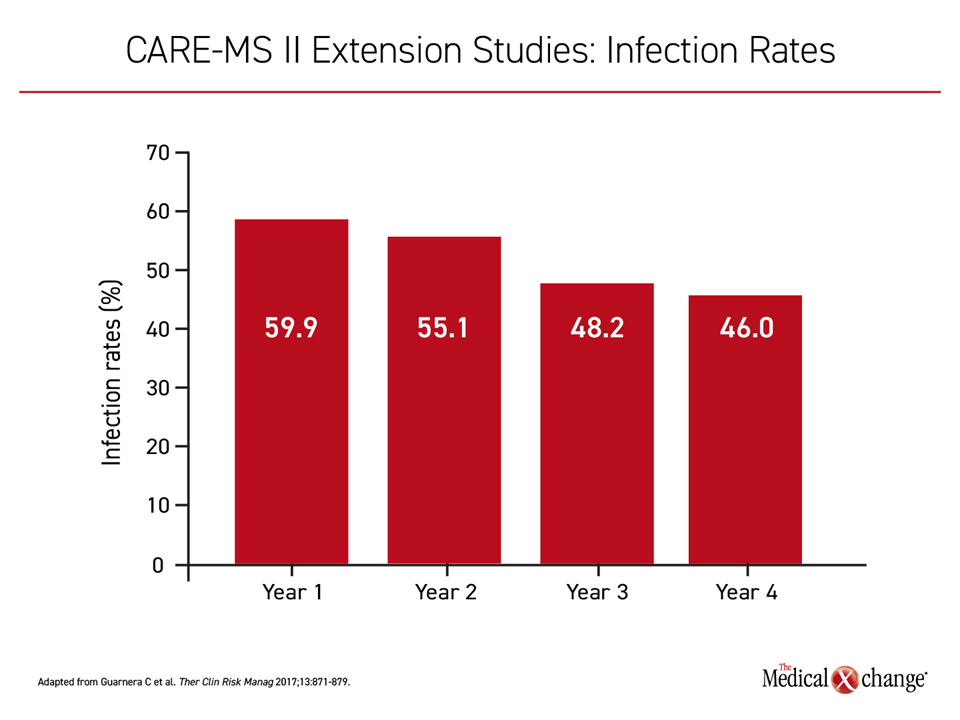
In the CARE-MS II trial, the majority of patients randomized to either arm developed an infection over the study period (77% on alemtuzumab vs. 66% on interferon beta-1a). Most involved the upper respiratory tract. Serious infections were uncommon on both therapies but slightly lower among those randomized to alemtuzumab (1% vs. 4%). In the CARE-MS II extension studies, infection rates on alemtuzumab fell for each year of study, declining from 59.9% in the first year to 46% in the fourth year (Figure 4). Due to an association between alemtuzumab and increased risk of viral infections including herpes virus and varicella zoster virus, patients should be vaccinated against preventable infectious diseases prior to initiating alemtuzumab. Rare infections in patients taking alemtuzumab, such as listeria meningitis and a pulmonary Nocardia beijingensis,29,30 have been reported, but none have been fatal.
Secondary autoimmune disorders, potentially linked to the repopulation of T cells after treatment, are associated with alemtuzumab. The most common involve the thyroid gland, producing such complications as hyperthyroidism, hypothyroidism, and thyroiditis. In the CARE-MS II trial, these occurred in 16% of patients.15 Idiopathic thrombocytopenic purpura (ITP), which is also considered autoimmune related, was observed in 1% of patients. In the CAMMS223 trial,31 a case of ITP in a patient receiving alemtuzumab died of intracranial hemorrhage attributed to this complication. However, autoimmune complications are not typically difficult to manage in patients who are instructed to recognize and report symptoms.
The risks of alemtuzumab are substantial but generally manageable in patients monitored appropriately. Although these risks should not be underestimated, the benefit-to-risk ratio of this treatment might be considered favorable by many patients. This includes patients with a high degree of disease activity early in the course, a phenotype that correlates with accelerated disease progression.3,8,32-34 However, most RRMS patients face eventual progression. In the context of other features of an induction regimen, which, in the case of alemtuzumab, means treatment intervals a year apart, patients might reasonably select treatment with a low relative risk of early progression to disability.
Conclusion
The substantial increase in the number and types of treatments for RRMS has permitted a new level of individualization of care. Relative to the first-generation immunomodulators, high-efficacy agents, including oral drugs, reduce rates of relapse and delay disability. Relative to high-efficacy agents, therapies designed to deplete immune cells driving RRMS address the underlying pathophysiology. By eliminating immune cells in order to reprogram the inflammatory response, alemtuzumab has been associated with reversal rather than stabilization of disability as measured with EDSS. This effect is accompanied by an increased risk of infections and secondary autoimmune disorders, which can be serious but are manageable in patients instructed on recognizing signs and symptoms.
RRMS patients well educated about their disease and the available treatments should be encouraged to calculate a benefit-to-risk ratio for the available treatment options. First-generation immunomodulators are safe but offer limited efficacy. Escalation strategies have been a reasonable strategy to sequence the growing number of treatment options, but almost all RRMS patients progress eventually. Alemtuzumab is a reasonable option early or even initially in patients with features suggesting an aggressive disease course or who place a priority on avoiding physical disabilities and cognitive loss.
References
1. Lublin F. History of modern multiple sclerosis therapy. J Neurol 2005;252 Suppl 3:iii3-iii9.
2. Grand’Maison F, Yeung M, Morrow SA, et al. Sequencing of disease-modifying therapies for relapsing-remitting multiple sclerosis: a theoretical approach to optimizing treatment. Curr Med Res Opin 2018;34:1419-30.
3. Freedman MS, Selchen D, Prat A, Giacomini PS. Managing Multiple Sclerosis: Treatment Initiation, Modification, and Sequencing. Can J Neurol Sci 2018;45:489-503.
4. Rice GP, Hartung HP, Calabresi PA. Anti-alpha4 integrin therapy for multiple sclerosis: mechanisms and rationale. Neurology 2005;64:1336-42.
5. Fenu G, Lorefice L, Frau F, Coghe GC, Marrosu MG, Cocco E. Induction and escalation therapies in multiple sclerosis. Antiinflamm Antiallergy Agents Med Chem 2015;14:26-34.
6. Ontaneda D, Tallantyre E, Kalincik T, Planchon SM, Evangelou N. Early highly effective versus escalation treatment approaches in relapsing multiple sclerosis. Lancet Neurol 2019;18:973-80.
7. Claflin SB, Broadley S, Taylor BV. The Effect of Disease Modifying Therapies on Disability Progression in Multiple Sclerosis: A Systematic Overview of Meta-Analyses. Front Neurol 2018;9:1150.
8. Stankiewicz JM, Weiner HL. An argument for broad use of high efficacy treatments in early multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 2020;7.
9. Grand’Maison F, Yeung M, Morrow SA, et al. Sequencing of high-efficacy disease-modifying therapies in multiple sclerosis: perspectives and approaches. Neural Regen Res 2018;13:1871-4.
10. Scott LJ, Figgitt DP. Mitoxantrone: a review of its use in multiple sclerosis. CNS Drugs 2004;18:379-96.
11. Phillips JT, Giovannoni G, Lublin FD, et al. Sustained improvement in Expanded Disability Status Scale as a new efficacy measure of neurological change in multiple sclerosis: treatment effects with natalizumab in patients with relapsing multiple sclerosis. Mult Scler 2011;17:970-9.
12. Morrissey SP, Le Page E, Edan G. Mitoxantrone in the treatment of multiple sclerosis. Int MS J 2005;12:74-87.
13. McCormack PL. Natalizumab: a review of its use in the management of relapsing-remitting multiple sclerosis. Drugs 2013;73:1463-81.
14. Brandstadter R, Katz Sand I. The use of natalizumab for multiple sclerosis. Neuropsychiatr Dis Treat 2017;13:1691-702.
15. Coles AJ, Twyman CL, Arnold DL, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet 2012;380:1829-39.
16. Coles AJ, Cohen JA, Fox EJ, et al. Alemtuzumab CARE-MS II 5-year follow-up: Efficacy and safety findings. Neurology 2017;89:1117-26.
17. Giovannoni G, Cohen JA, Coles AJ, et al. Alemtuzumab improves preexisting disability in active relapsing-remitting MS patients. Neurology 2016;87:1985-92.
18. Schippling S, Pelletier D, Barnett M, Boster A, Comi G. Alemtuzumab reduced MRI lesions and the rate of brain volume loss in CARE-MS II patients switching from SC IFNB-1a: 5-year follow-up (TOPAZ studyP). Neurology 2018;90:P5.301.
19. Alrouighani R, Singer BA, Broadley S, Eichau S, Hartung HP. Alemtuzumab improves clinical and MRI disease activity outcomes, including slowing of brain volume loss, iin relapsing-remitting multiple sclerosis patients over 8 years: CARE-MS II follow-up (TOPAZ study). Mult Scler Relat Disord 2018;26:258 (P609).
20. Giovannoni G, Comi G, Cook S, et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med 2010;362:416-26.
21. Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus Interferon Beta-1a in Relapsing Multiple Sclerosis. N Engl J Med 2017;376:221-34.
22. Le Page E, Edan G. Induction or escalation therapy for patients with multiple sclerosis? Rev Neurol (Paris) 2018;174:449-57.
23. Le Page E, Leray E, Taurin G, et al. Mitoxantrone as induction treatment in aggressive relapsing remitting multiple sclerosis: treatment response factors in a 5 year follow-up observational study of 100 consecutive patients. J Neurol Neurosurg Psychiatry 2008;79:52-6.
24. Cocco E, Marrosu MG. The current role of mitoxantrone in the treatment of multiple sclerosis. Expert Rev Neurother 2014;14:607-16.
25. Cossburn M, Pace AA, Jones J, et al. Autoimmune disease after alemtuzumab treatment for multiple sclerosis in a multicenter cohort. Neurology 2011;77:573-9.
26. Cornaby C, Gibbons L, Mayhew V, Sloan CS, Welling A, Poole BD. B cell epitope spreading: mechanisms and contribution to autoimmune diseases. Immunol Lett 2015;163:56-68.
27. Menon S, Shirani A, Zhao Y, et al. Characterising aggressive multiple sclerosis. J Neurol Neurosurg Psychiatry 2013;84:1192-8.
28. Tallantyre EC, Major PC, Atherton MJ, et al. How common is truly benign MS in a UK population? J Neurol Neurosurg Psychiatry 2019;90:522-8.
29. Sheikh-Taha M, Corman LC. Pulmonary Nocardia beijingensis infection associated with the use of alemtuzumab in a patient with multiple sclerosis. Mult Scler 2017;23:872-4.
30. Rau D, Lang M, Harth A, et al. Listeria Meningitis Complicating Alemtuzumab Treatment in Multiple Sclerosis–Report of Two Cases. Int J Mol Sci 2015;16:14669-76.
31. Investigators CT, Coles AJ, Compston DA, et al. Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N Engl J Med 2008;359:1786-801.
32. Fernandez O. Is there a change of paradigm towards more effective treatment early in the course of apparent high-risk MS? Mult Scler Relat Disord 2017;17:75-83.
33. Rush CA, MacLean HJ, Freedman MS. Aggressive multiple sclerosis: proposed definition and treatment algorithm. Nat Rev Neurol 2015;11:379-89.
34. Rudick RA, Lee JC, Cutter GR, et al. Disability progression in a clinical trial of relapsing-remitting multiple sclerosis: eight-year follow-up. Arch Neurol 2010;67:1329-35.