Cardiology
European Society of Cardiology (ESC) Congress 2020
ARIADNE Real-World Study: Confirming Outcomes and Wellbeing of Patients with Chronic Heart Failure
Virtual Meeting – The large heart failure ARIADNE registry has associated an angiotensin receptor neprilysin inhibitor (ARNI) with substantial benefit in the real-world setting. After 12 months of follow-up in patients enrolled with reduced ejection fraction (HFrEF) heart failure, almost half improved in heart failure class, and more than one third achieved a meaningful improvement in quality of life.
“Throughout follow-up in this real-world study, symptoms and quality of life improved with the addition of the ARNI,” reported Dr. Aldo Pietro Maggioni, Associazione Nazionale Medici Cardiologi Ospedalieri (ANMCO) Research Center, Florence, Italy.
For a related eLearning program on heart failure remodeling with Dr. Kim Connelly, click here
Several randomized trials, including PARADIGM-HF and PIONEER-HF, have demonstrated a mortality benefit when sacubitril/valsartan was added to standard therapy in heart failure. Hospitalizations for heart failure and progressive cardiovascular remodeling are also reduced, while quality of life has improved. In this large registry, called ARIADNE, outcomes and wellbeing in HFrEF patients were confirmed in routine clinical practice.
“The ARIADNE registry provides real-world information about the efficacy of sacubitril/valsartan as administered in an outpatient setting,” said Dr. Maggioni, who presented data on 9069 HFrEF patients followed for 12 months in 17 European countries. Of these, 52.5% received sacubitril/valsartan at baseline. The remaining patients did not receive this treatment despite recommendations, and continued on their previous individualized heart failure medication.
Consistent with the pivotal trials, those who received sacubitril/valsartan had lower total mortality (3.8% vs. 5.0%), lower cardiovascular mortality (1.8% vs. 2.2%) and fewer catheterization or angiography procedures (1.7% vs. 2.8%) over the course of the 12-month follow-up.
The proportion of patients in NYHA class III or IV treated with sacubitril/valsartan fell from 44.6% to 24.0% by the end of 12 months. In those not taking the ARNI, no reduction was reported.
Similarly, 36.2% of patients on sacubitril/valsartan achieved a clinically-meaningful improvement (≥5 points) in the Kansas City Cardiomyopathy Questionnaire (KCCQ) score, a degree of change that was not reported in patients not placed on this guideline-recommended treatment.
In an ARIADNE safety analysis presented separately at the 2020 ESC Congress, only 4.1% of patients discontinued sacubitril/valsartan as a result of an adverse event over the 12-month follow-up.
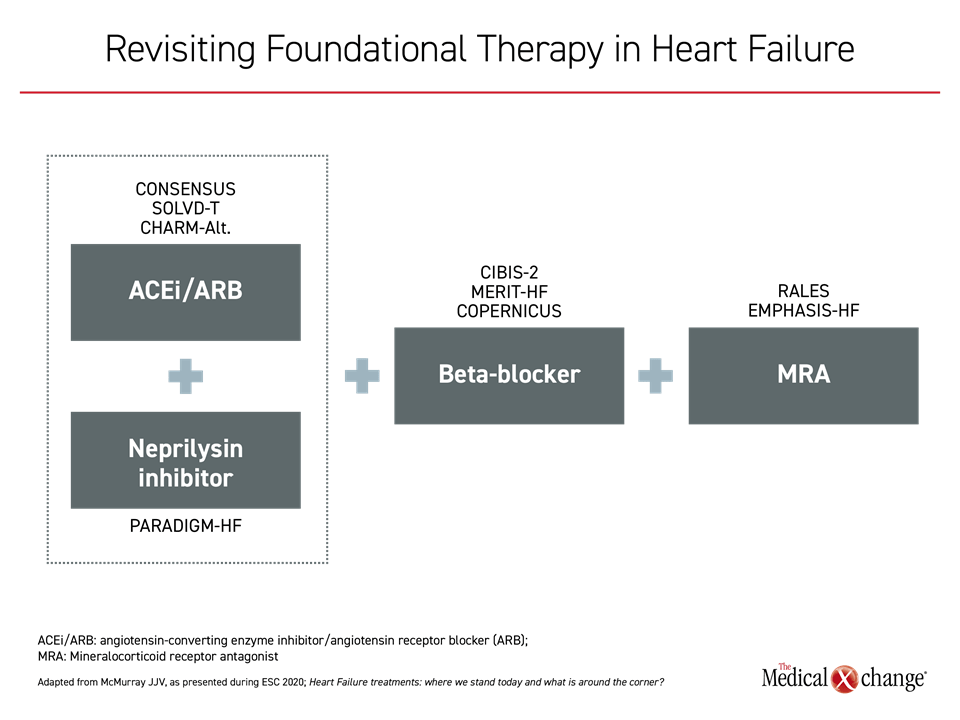
In a symposium at the ESC Congress 2020, the principal investigator of the pivotal PARADIGM-HF study, Dr. John J.V McMurray, Professor of Cardiology, University of Glasgow, UK, reiterated that sacubitril/valsartan should now be considered one of the “foundational” therapies, a designation used to identify the four therapies that conferred a mortality benefit in large multinational randomized trials (Figure 1). All of these therapies are part of guideline-based treatment.
Conclusion
In addition to substantiating an improvement in survival in the real-world setting, the ARIADNE registry also documents immediate benefits to patients in the form of improved function and quality of life.