Dermatology
29th Virtual Congress of the European Academy of Dermatology and Venereology (EADV)
Control of Pathology Beyond the Skin Might Best Characterize Benefit of Psoriasis Therapy
Virtual Meeting – In the management of psoriasis, clear skin is often the immediate goal for patients, but clinicians should consider the larger goal of suppressing the systemic inflammation that drives disease progression, according to data from the EADV 2020 Congress. Disease control at the level of the skin is the first step, but inflammation control has important implications for long-term outcome. Biologics have not been shown to be comparable for modifying an upregulated immune system that mediates disease beyond the skin. Even among biologics effective for skin clearing, systemic upregulation of inflammation is relevant to long-term risks.
Importantly, many if not most psoriasis patients have involvement beyond the skin, according to Dr. Alice Gottlieb, Clinical Professor of Dermatology, Icahn School of Medicine at Mount Sinai, New York. Presenting a comparative study that reinforced this point, Dr. Gottlieb said, “Imaging studies using musculoskeletal ultrasound have shown that up to half of patients with psoriasis limited to the skin exhibit inflammatory and structural abnormalities in their joints and entheses.”
“Imaging studies using musculoskeletal ultrasound have shown that up to half of patients with psoriasis limited to the skin exhibit inflammatory and structural abnormalities in their joints and entheses.”
EXCEED Trial and Limiting Progression
In the comparative analysis, adalimumab, a monoclonal antibody that targets TNF, was compared to secukinumab, a monoclonal antibody that neutralizes interleukin-17a (IL-17a). The data were drawn from the phase 3b head-to-head EXCEED trial, which randomized 853 patients with psoriatic arthritis (PsA) to adalimumab or secukinumab.
The focus of this prespecified analysis of EXCEED was the subset of 211 patients with moderate to severe plaque and nail psoriasis with greater than 10% body surface area (BSA) involvement or a Psoriasis Area and Severity Index (PASI) of at least 10. Comparisons at 52 weeks were made for ACR50/PASI 100 as well as ACR20, ACR70, PASI 90, Dermatology Life Quality Index (DLQI), and Minimal Disease Activity (MDA) score.
“Secukinumab provided numerically higher responses compared to adalimumab in skin, musculoskeletal, and composite indices, as well as simultaneous improvement in both,” Dr. Gottlieb reported. She further reported that these differences translated into a quality of life advantage for secukinumab. It also supported the larger premise that drug efficacy beyond control of skin lesions might be important early in disease due to the substantial risk of progression.
Psoriatic Joint Involvement Often Subclinical
“Approximately one-third of biologic-treated patients with psoriasis will eventually develop PsA,” Dr. Gottlieb said. From the subclinical perspective when structural abnormalities are captured on imaging, the rates are substantially higher, she added. Based on the observation that “early diagnosis and treatment of PsA has been linked to improved outcomes,” Dr. Gottlieb indicated that the relative efficacy of biologics on system inflammatory processes is relevant to long-term outcomes.
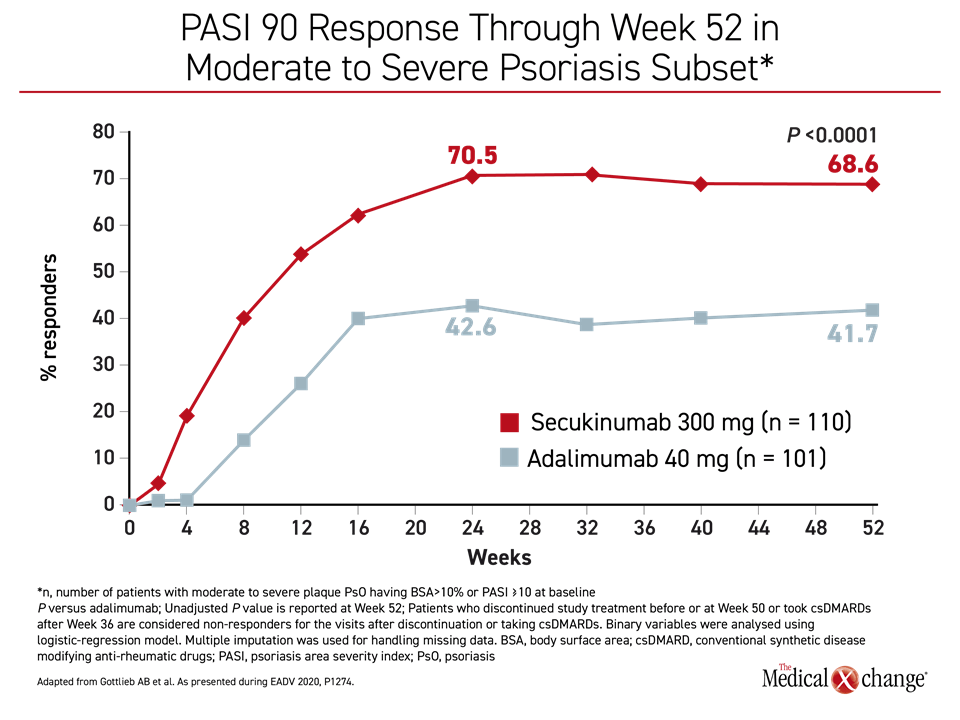
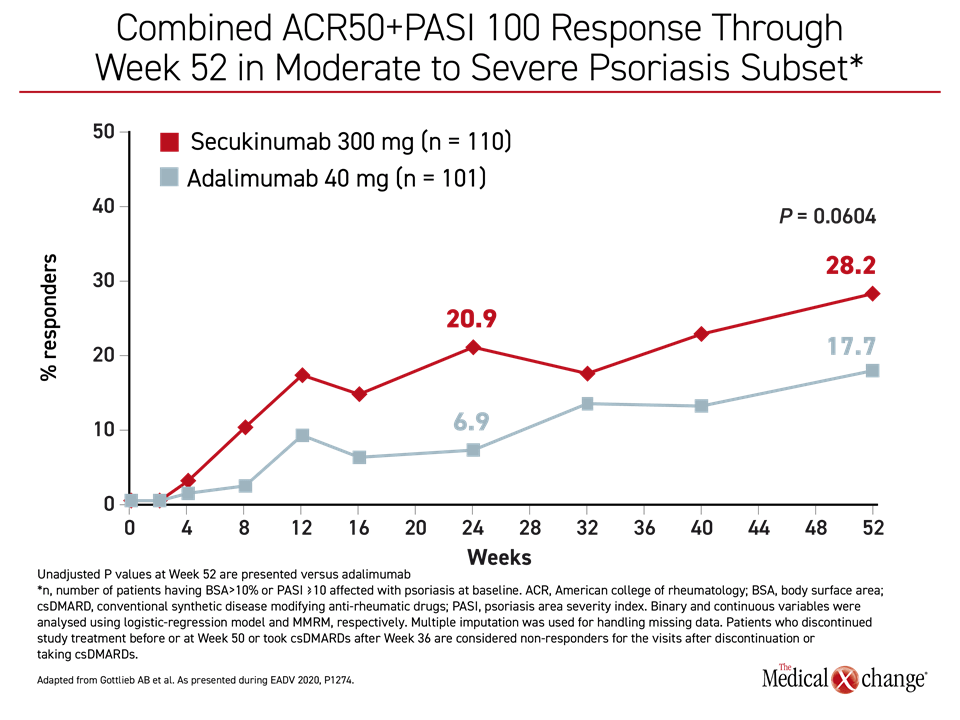
At 52 weeks, the proportion of patients who achieved a PASI 90 was significantly higher (P<0.0001) among those randomized to secukinumab relative to adalimumab, and the combined American College of Rheumatology (ACR)50 and PASI 100 response approached statistical significance (P=0.0604) (Figure 1) and (Figure 2). PASI 75 (87.2% vs. 59.6%; P<0.0001) and the PASI 100 responses (39.1% vs. 23.8%; P=0.0138) were also significantly greater for secukinumab at 52 weeks. The clinical significance of these scores favoring secukinumab was reflected in the greater improvement in DLQI for those randomized to secukinumab (-10.27 vs. -8.32; P=0.0137).
For outcomes where differences did not reach statistical significance, such as disease activity in PsA (DAPSA), remission (73.6% vs. 59.9%; P=0.0733) and resolution of enthesitis (74.5% vs. 66.2%; P=0.1571), the majority favored secukinumab numerically.
Prevention or resolution of enthesitis is a key concept in a strategy that places an emphasis on selecting therapies for psoriasis that have an impact on disease beyond the skin. The prospective IVEPSA study, published in 2019, has already generated evidence that treating patients with psoriasis at high risk of progression prevents or reduces the risk of joint involvement, according to Dr. Gottlieb.
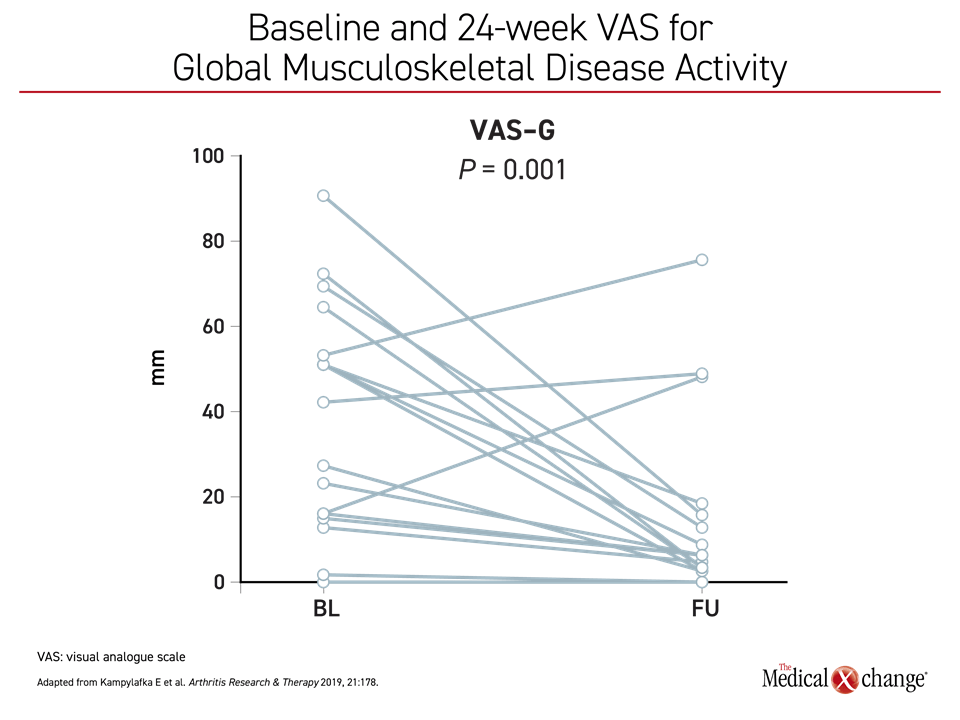
In the IVEPSA study, 20 psoriasis patients with arthralgia, about half of whom had tender joints, were followed for 24 weeks on secukinumab (Kampylafka E et al. Arthritis Res Ther 2019; 21:178). Consistent with conventional psoriasis trials with biologics, total PASI (P=0.002) and BSA (P=0.003) declined significantly from baseline following initiation of secukinumab, but there was also rapid and steep declines in symptoms relevant to joint involvement. These not only included improvement in the Visual Analog Scale (VAS) for pain (P=0.004) but also for global musculoskeletal disease activity (Figure 3).
These are consistent with growing evidence that patients with psoriasis can be protected from the effects of chronic systemic inflammation, including joint involvement and progression, if the disease process is controlled at an early stage, noted Dr. Gottlieb. She is now involved in a double blind, placebo-controlled trial called INTERCEPT that will further test this hypothesis. This trial is enrolling patients with moderate to severe plaque psoriasis who are required to have sonographic evidence of enthesitis on screening but no prior diagnosis of PsA.
“These are consistent with growing evidence that patients with psoriasis can be protected from the effects of chronic systemic inflammation, including joint involvement and progression, if the disease process is controlled at an early stage.”
New Study Tests Early Enthesitis Treatment
“Although the early diagnosis and treatment of PsA has been linked to improved patient outcomes in previous studies, the effect of systemic therapies on subclinical changes has not been well studied,” Dr. Gottlieb explained. In the INTERCEPT study, the goal is to measure the impact of secukinumab relative to placebo on change from baseline in an objective measure of enthesitis over the 16 weeks.
She characterized this as the first randomized study to test the impact of a biologic on subclinical joint symptoms in patients with psoriasis. Changes from baseline will be documented with the Outcome Measures in Rheumatology (OMERACT) ultrasound enthesitis score. It is one of the most significant attempts so far to further explore the value of reducing chronic system inflammation to block progression of psoriasis to organ systems beyond the skin.
The relationship of chronic systemic inflammation in patients with psoriasis to non-skin-related health risks has attracted the attention of major research centers, including the National Heart, Lung, and Blood Institute (NHLBI) in the United States. At the EADV 2020 Congress, a research team led by Dr. Nehal M. Mehta, who directs a laboratory for the study of inflammation in the NHLBI’s cardio-pulmonary branch, looked at the impact of secukinumab on inflammatory biomarkers, particularly high-sensitivity C-reactive protein (hsCRP).
Cardiovascular Disease Link to Psoriasis Explored
“Psoriasis is a chronic systemic inflammatory disease that has now been associated with other diseases in which inflammation is implicated, including cardiovascular (CV) disease,” explained Dr. Mehta. He noted that the same biomarkers associated with CV disease, such as hsCRP and GlycA are also increased in patients with psoriasis.
“Psoriasis and CV conditions share pathological inflammatory mechanisms including increased expression of IL-17A, which could be a mechanistic link between these diseases,” he said.
In this study, the effect of secukinumab on systemic inflammatory markers was evaluated in patients with psoriasis who were on an extended course of secukinumab. The data were drawn from a secukinumab database with 4,742 patients and evaluated changes over time in those in a high-risk CV group, defined as a baseline hsCRP >4 mg/L, as well as the entire study population.
“Psoriasis and CV conditions share pathological inflammatory mechanisms including increased expression of IL-17A.”
Reductions in hsCRP Are Large and Sustained
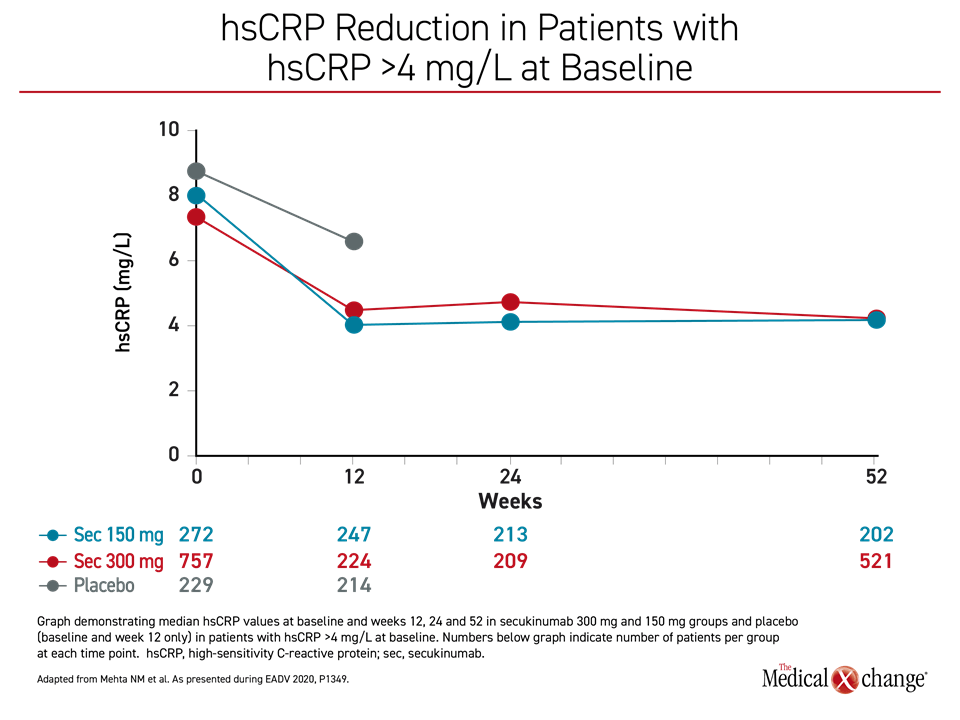
In the high-risk CV group, hsCRP levels fell modestly within the initial 12 weeks of monitoring in the placebo group but more precipitously among those randomized to either the 150 mg or the 300 mg dose of secukinumab (Figure 4). This reduction was not only maintained out to 52 weeks among those treated with secukinumab, but it then remained suppressed when follow-up was extended to two years. Other inflammatory biomarkers, such as the neutrophil-to-lymphocyte ratio (NLR) also fell rapidly and remained suppressed over the course of follow-up, according to Dr. Mehta.
In the overall study population, hsCRP was also reduced, but the reduction was more marked in those who started therapy with an elevated hsCRP, Dr. Mehta reported. The reduction in NLR, which has been associated with increased CV mortality among those with a ratio of 2.5 or higher, also remained stably suppressed in the physiologic range. Secukinumab had a neutral effect on traditional CV risk factors, such as serum lipids, blood pressure, and body mass index, which were also evaluated over the study period.
The findings associate secukinumab with a “salutary anti-inflammatory effect as measured with CV biomarkers,” Dr. Mehta concluded. Due to the established relationship between psoriasis and increased CV risk, Dr. Mehta said these observations “should be followed up with randomized coronary vascular imaging and CV event studies.”
ObePso-S Study of Inflammatory Proteins
The same principle was the basis of a similar study presented at this year’s EADV Congress. Called the ObePso-S study, the goal was to explore “the known overlap in inflammatory pathways activated in psoriasis and cardiometabolic disease,” according to Dr. James G. Kreuger, Head of the Laboratory for Investigative Dermatology, Rockefeller University, New York.
In this phase 4 study, 82 patients with plaque psoriasis were randomized in a 2:1 fashion to 300 mg secukinumab administered at standard dosing intervals or placebo and followed for 12 weeks. A group of 17 healthy volunteers were followed simultaneously. Lesional and non-lesional punch skin biopsies performed at baseline, week 12, and week 52, were evaluated for expression of inflammatory proteins.
Change in Proteins Correlate with PASI Scores
At baseline, PASI scores were correlated with several proteins associated with inflammation, including IL-17A, IL-17C, IL-20 and MMP3. At week 12, the change in PASI scores among those treated with secukinumab was again correlated with all of these inflammatory proteins as well as IL-12B and IL-6. At week 52, reductions in the expression of several of these proteins, including IL-17C, IL-20, and P13 were sustained.
In this study, secukinumab was effective in clearing psoriasis plaques, which was also monitored, but Dr. Kreuger emphasized the main result, which was a “positive systemic effect on the expression of inflammatory proteins, normalizing the production of numerous markers of inflammation and CV risk.” Most of the inflammatory proteins associated with both psoriasis and CV risk were “stably improved through 52 weeks” on secukinumab, he added.
There are other data demonstrating efficacy differences between biologics for the treatment of psoriasis. Newly-pooled data from the CLEAR and CLARITY studies, which compared secukinumab to the IL-12/IL-23 inhibitor ustekinumab for the treatment of psoriasis, associated secukinumab with about a 15% therapeutic gain for PASI 90 at week 52, according to Dr. Blauvelt, President, Oregon Medical Research Center, Portland, who presented these data at the EADV Congress.
There was not only a greater magnitude of improvement on secukinumab than ustekinumab at 52 weeks, but targeting IL-17A “led to more rapid and greater sustained improvement,” Dr. Blauvelt reported.
Clearing of the skin is important, but the relative value of targeted therapies might be best considered on the degree to which they suppress chronic systemic inflammation. For therapies effective against mediators of inflammation implicated in progression of psoriasis to PsA or shared by other pathology related to inflammation, such as CV disease, biologic targets might not be interchangeable as suggested by the EADV Congress data.
Conclusion
For patients with moderate-to-severe psoriasis, biologic therapies have represented a major advance for clearing or nearly clearing psoriatic plaques, but psoriasis is the cutaneous manifestation of a systemic inflammatory process. Clearing of the skin is an urgent immediate goal for patients, but downregulating the inflammatory response is central to long-term control of the underlying disease, including reducing the risk of relapse, preventing progression to joint inflammation, and potentially for prevention of other complications of dysregulated immune function.