Rheumatology
American College of Rheumatology (ACR) Convergence 2020
SELECT-PsA 1 & SELECT-PsA 2: Oral Targeted Therapy for PsA Promises Efficacy in Bio-Naïve and Refractory Patients
Virtual Meeting – In patients with psoriatic arthritis (PsA), an oral targeted JAK inhibitor, already approved for rheumatoid arthritis, is effective even in those who have failed multiple biologics, according to data presented at ACR Convergence 2020. This newer agent has been found superior to a TNF inhibitor following inadequate response to a conventional synthetic disease-modifying antirheumatic drug (csDMARD). In expanded data from two phase 3 trials that enrolled patients who failed one or more prior biologic DMARDs (bDMARD), complete or near complete responses were seen in a relatively high rate of patients, as was a rapid and sustained response in pain relief.
The main results of the phase 3 SELECT-PsA 1 and SELECT-PsA 2 trials on the JAK inhibitor upadacitinib were reported earlier this year, but the updated results at the ACR Convergence 2020 confirm an unusual degree of disease control across joint and skin involvement regardless of prior therapy. In particular, relatively high rates of control were seen whether bDMARD-experienced patients were previously exposed to a TNF inhibitor, an anti-IL-17 monoclonal antibody, or both. Further, new data also show the efficacy of upadacitinib to be about the same if used as monotherapy or in combination with csDMARDs. Safety findings were consistent with the known safety profile and no new risks were identified in these latest results.
Benefit Shown Regardless of Prior Failed Treatment
“The efficacy of upadacitinib in treating musculoskeletal symptoms, psoriasis, physical function, fatigue, and impaired quality of life in patients with inadequate response to bDMARDs was generally consistent whether response was inadequate to one or to multiple prior agents,” reported Dr. Philip J. Mease, Director of Rheumatology Research, Swedish Medical Center, Seattle, Washington.
Comprehensive disease control, including pain reduction, in treatment-experienced PsA patients was a recurring message from the SELECT-PsA 1 and SELECT-PsA 2 data. The SELECT-PsA 1 trial enrolled patients with an inadequate response to csDMARDs and SELECT-PsA 2 randomized patients with an inadequate response to bDMARDs. In both, the steep reductions in joint and skin symptoms permitted many patients to achieve minimal disease activity (MDA), a rigorous measure of clinical benefit in this multifocal rheumatic disease.
Quick Response across PsA Clinical Domains
“The efficacy was observed in all core clinical domains of PsA,” confirmed Dr. Mease, who delivered several sets of data generated by the SELECT-PsA 2 trial. He emphasized that responses were rapid. For the major domains of PsA, symptom reductions were often significant relative to placebo by the end of the second week of the trial.
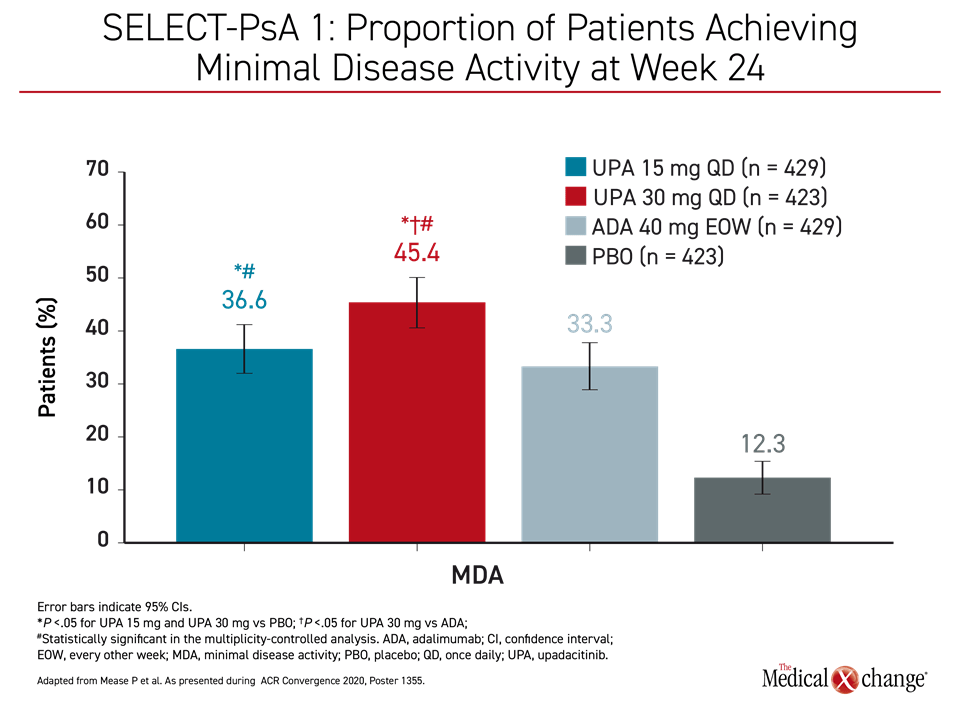
Responses were also rapid in SELECT-PsA 1, which included adalimumab and placebo arms. According to the updated data presented by Dr. Iain B. McInnes, Chair of Medicine, University of Glasgow, UK, the proportion of patients with MDA at week 24 approached 50% for the higher dose of upadacitinib and nearly 40% for the lower dose (Figure 1).
SELECT-PsA 1 Design and Results
In the SELECT-PsA 1 trial, 1704 patients were randomized to 15 mg once-daily upadacitinib, 30 mg once-daily upadacitinib, 40 mg of adalimumab injected every other week, or placebo (McInnes IB et al. ACR Convergence 2020, abstract 2026). An inadequate response to at least one csDMARD was an entry criterion. At entry, patients had a mean tender joint count of approximately 20 and a mean swollen joint count of 11. The mean baseline Psoriasis Area and Severity Index (PASI) was slightly greater than 11.
For the higher dose of upadacitinib, the lower dose of upadacitinib, adalimumab, and placebo, the proportions of patients achieving the primary endpoint of ACR20 by week 24 were 79%, 71%, 65%, and 36%, respectively.
“In terms of the ACR20 response at week 12, both doses of upadacitinib achieved non-inferiority to adalimumab, but the 30 mg was superior [P<0.001],” Dr. McInnes reported.
The proportion of patients achieving PASI75 was 63%, 62%, 53% and 21% for the same four groups. For this endpoint, the degree of response for upadacitinib relative to adalimumab was significant for both the higher dose (P<0.001) and lower dose (P<0.05).
“In terms of the ACR20 response at week 12, both doses of upadacitinib achieved non-inferiority to adalimumab, but the 30 mg was superior.”
Radiographic progression as measured by joint space narrowing or erosion was significantly reduced on both doses of upadacitinib relative to placebo at the end of 24 weeks. For several measures, such as the Health Assessment Questionnaire-Disability Index (HAQ-DI) and the 36-item Short Form Physical Component Summary (SF-36 PCS), the impact of PsA was significantly reduced with either the 30 mg or 15 mg dose of upadacitinib relative to adalimumab.
SELECT-PsA 2 Design and Results
In SELECT-PsA 2, inadequate response to a bDMARD was an entry criterion. According to data presented (Genovese M et al. ACR Convergence 2020, abstract 0504), nearly 40% of those enrolled had failed at least two bDMARDs. The 641 PsA patients who participated were randomized to 15 mg once-daily upadacitinib, 30 mg once-daily upadacitinib, or placebo. The mean baseline tender joint count was approximately 25 and the mean swollen joint count was 12. The mean baseline PASI score slightly exceeded 10.0.
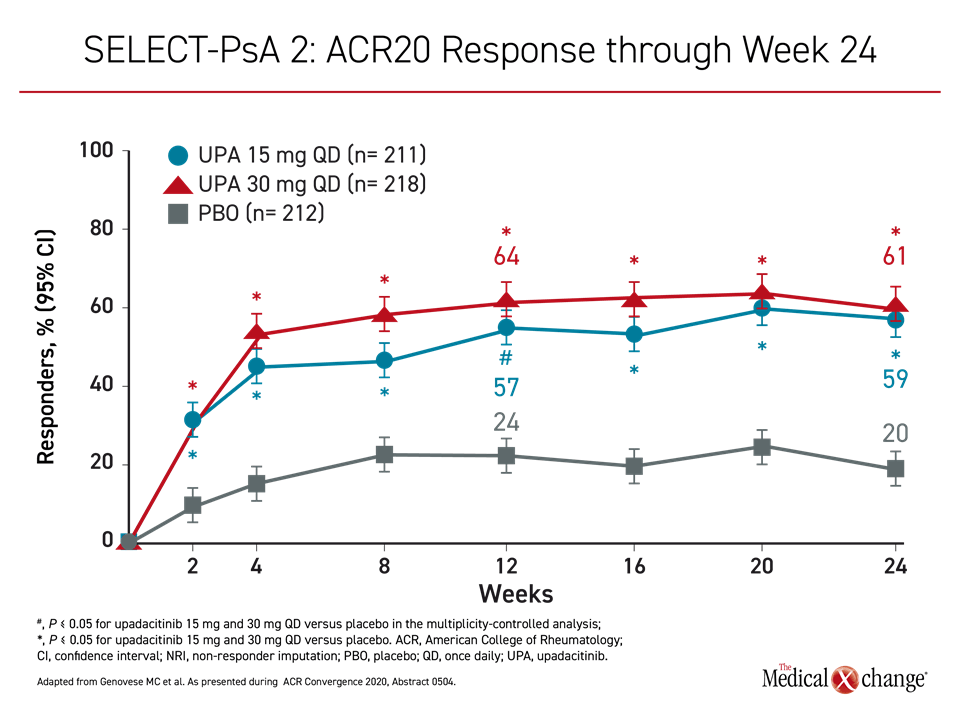
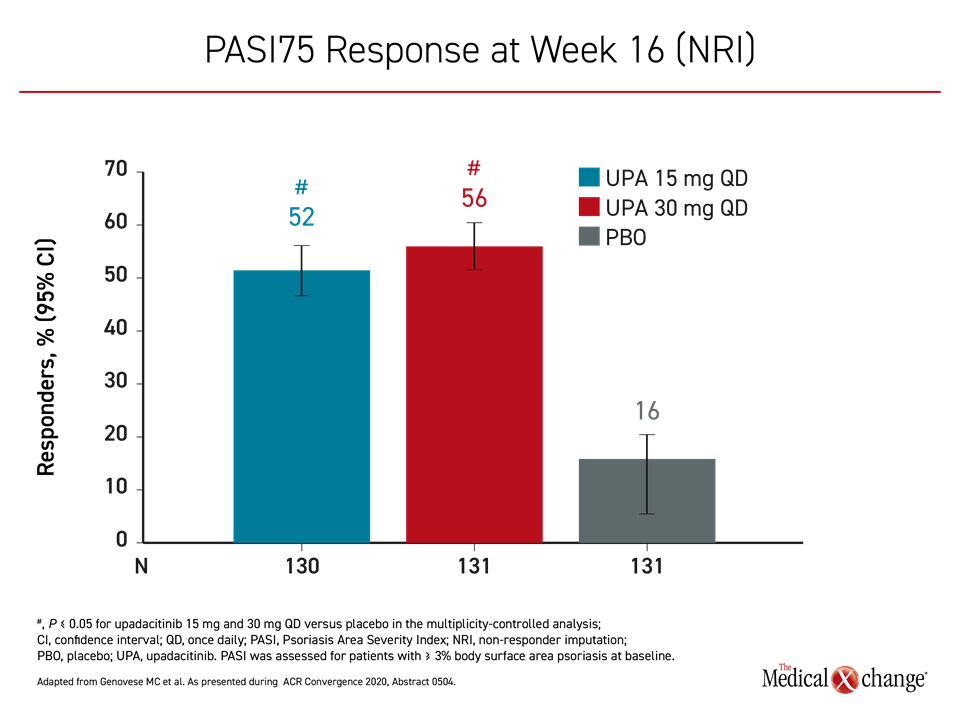
For the 30 mg upadacitinib, 15 mg upadacitinib, and placebo groups, the proportions of patients achieving the primary endpoint of ACR20 by week 12 were 64%, 57%, and 24%, respectively (Figure 2). The ACR50 scores at week 24 were 38%, 32%, and 5%, respectively. The proportion of patients achieving a PASI75 by week 16 was 56%, 52%, and 16%, respectively (Figure 3).
Achieved early and then sustained out to 24 weeks, “clear or almost clear skin was observed in about 40% of patients,” reported Dr. Mease.
Due to the substantial response rates for both joint and skin symptoms in a difficult population refractory to bDMARDs, 29% of those on the higher dose and 25% of those on the lower dose of upadacitinib achieved MDA.
Results Characterization
The moderator of the ACR session in which these data were presented, Dr. Christopher Ritchlin, Professor of Medicine, University of Rochester School of Medicine, New York, was impressed by the relatively high rate of complete or near complete responses in this heavily-treated population. He asked Dr. Mease to speculate on why these effects were so “dramatic.”
“We see the direct effects [of upadacitinib] on the JAK pathway, but we are also seeing a striking amount of inhibition of cytokines not directly affected by this drug, including TNF and IL-17,” Dr. Mease said. He noted that this “crosstalk” leading to downregulation of inflammatory mediators might be additive to the selectivity that upadacitinib exhibits for JAK1 relative to other proteins in the JAK family.
In a detailed analysis of response relative to prior bDMARD exposure, which was presented separately (Mease P et al. ACR Convergence 2020, abstract 1356), the efficacy of upadacitinib was remarkably consistent whether patients had failed just one prior bDMARD when compared to two or more or if patients had been treated with a TNF inhibitor, an IL-17 inhibitor, or both. For example, the rate of MDA among those treated with two prior bDMARDs was only modestly lower on the 15 mg dose of upadacitinib (20%) relative to one prior bDMARD exposure (23.3%). For those who received 2 or more bDMARDs to those who received just 1, the rate of MDA, although lower, remained meaningful for both the 15 mg (16%) and 30 mg doses (20.6%).
“We see the direct effects… on the JAK pathway, but we are also seeing a striking amount of inhibition of cytokines not directly affected by this drug, including TNF and IL-17.”
Overall, “the proportion of patients on upadacitinib achieving comprehensive disease control as measured by MDA was generally comparable regardless of number and type of prior bDMARDs,” Dr. Mease reported.
Treat-To-Target: Low Disease Activity
In another combined post-hoc analysis of SELECT-PsA 1 and SELECT-PsA 2 (Mease P et al. ACR Convergence 2020, abstract 1355), the goal was to assess the value of upadacitinib in a treat-to-target context. In addition to MDA, the proportion of patients achieving remission was calculated for very low disease activity (VLDA) scores, Disease Activity in Psoriatic Arthritis (DAPSA) scores, and Psoriatic Arthritis Disease Activity Score (PASDAS).
“In both the non-bDMARD patients and the more refractory bDMARD patients, treatment with upadacitinib 15 mg or 30 mg led to higher rates of remission or low disease activity as measured by these disease activity measures,” Dr. Mease said.
In SELECT-PsA 1, for example, DAPSA low disease activity was achieved at 24 weeks by 55.6% in the
30 mg upadacitinib group, 47.6% of the 15 mg upadacitinib group, 46.2% of the adalimumab group, and 16.5% of the placebo group. The advantage for 30 mg upadacitinib over adalimumab was significant (P<0.05). In SELECT-PsA 2, this same outcome was reached at 24 weeks by 41.7% of the 30 mg upadacitinib group, 34.6% of the 15 mg upadacitinib group, and 6.6% of placebo patients.
Treatment Effects of Monotherapy and Combination Therapy
Of patients randomized to upadacitinib in SELECT-PsA 1 and SELECT-PsA 2, 18% and 54%, respectively, were treated with upadacitinib alone. A subgroup analysis compared outcomes between the 574 patients treated with monotherapy to the 1342 who received upadacitinib plus a csDMARD. The treatment effects were slightly higher but not significantly different across major endpoints for both joint and skin involvement among those who also received a csDMARD.
“The treatment effects were generally consistent with overlapping confidence intervals for those on monotherapy relative to combination therapy,” reported Dr. Peter Nash, Director of the Rheumatology Research Unit, Griffith University, Brisbane, Australia.
This might be relevant to how this therapy is used in routine practice because “the frequency of serious infections and hepatic disorders were lower with upadacitinib monotherapy, particularly for the 15-mg dose of upadacitinib,” Dr. Nash reported. He suggested the results from this analysis “support the use of upadacitinib with or without concomitant non-biologic DMARDs.”
Pain Control Obtained Rapidly and Sustained
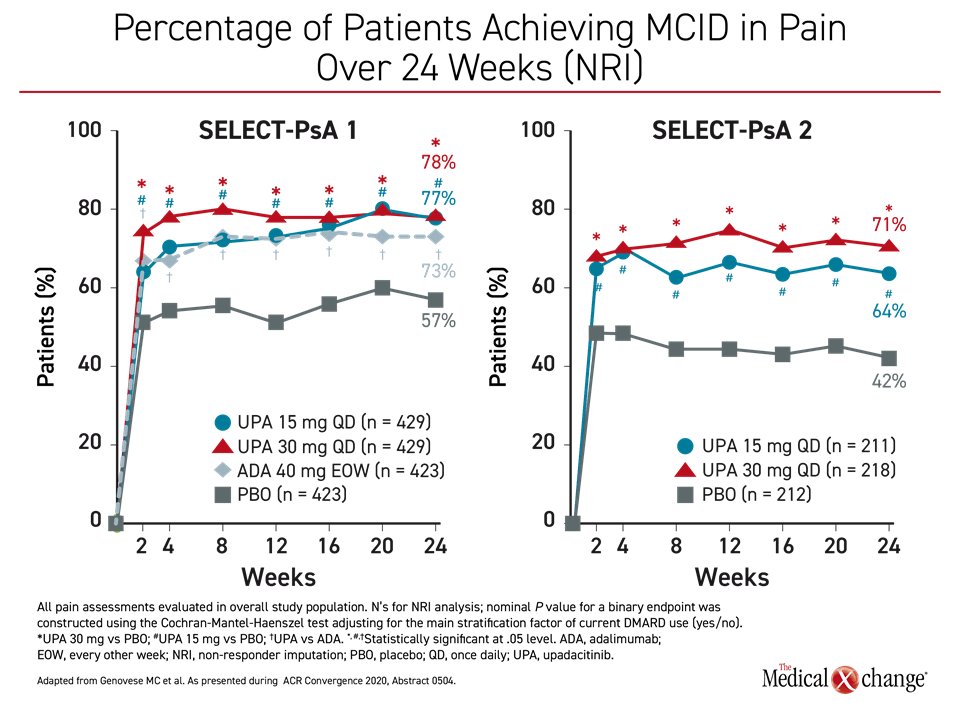
Pain control was also the focus of a separate analysis of the SELECT-PsA 1 and SELECT-PsA 2 trials (McInnes IB et al. ACR Convergence 2020, abstract 0896). As in the control of joint and skin involvement, a minimal clinically important difference (MCID) in pain defined as at least a 15% reduction in pain on the patient’s global assessment (PGA), was achieved within two weeks in the vast majority of patients on active therapy in SELECT-PsA 1 or either dose of upadacitinib in SELECT-PsA 2 (Figure 4). Pain relief of 70% or greater was achieved in 34% and 31% of the 30 mg and 15 mg upadacitinib patients, respectively, versus 25% of adalimumab and 9% of placebo patients.
In SELECT-PsA 1, the advantage for the higher dose relative to adalimumab was significant by week 2, but “improvements in most pain assessments were also higher with upadacitinib 15 mg versus adalimumab at week 24,” Dr. McInnes reported. As pain “is a dominant symptom of PsA and reduction of pain is a priority,” Dr. McInnes suggested these analyses further support the value of upadacitinib in patients with inadequate response to current treatments, including bDMARDs.
Findings Consistent with Known Safety Profile
Safety analyses from the two phase 3 trials have been reassuring (Burmester G et al. ACR Convergence 2020, abstract 1350). There was a higher rate of serious infection on the 30 mg dose of upadacitinib (2.7%) relative to adalimumab (0.7%) or placebo (0.8%), but the infection rate was not elevated in the 15 mg upadacitinib group (0.9%). Venous thromboembolism (VTE) rates were not different for the 30 mg dose of upadacitinib (0.2%) relative to adalimumab (0.5%), placebo (0.2%) or the 15 mg dose upadacitinib (0.2%). There were no major adverse cardiovascular events in the 30 mg group but two in the adalimumab group and one in the placebo group.
“The frequency of adverse events was higher with 30 mg upadacitinib compared with adalimumab but the safety profiles of 15 mg upadacitinib and adalimumab were generally similar,” reported Dr. Gerd R. Burmester, Department of Rheumatology, Charité University Hospital, Berlin, Germany.
Conclusion
Based on the data from the SELECT-PsA 1 and SELECT-PsA 2 phase 3 trials, the JAK inhibitor upadacitinib achieves a high rate of sustained response in PsA patients who have failed conventional or biologic DMARDs. The substantial rates of response even among patients who have not achieved an adequate response to more than two bDMARDs suggest that this agent will be an important addition to therapeutic options if these trials lead to regulatory approval. Relative to injectable biologics, a highly-active oral therapy is likely to be attractive to patients who are not well controlled on non-biologic DMARDs. The efficacy of a highly-selective JAK inhibitor in patients who fail a bDMARD or multiple bDMARDs with different mechanisms of action will fulfill an unmet clinical need.