Neurology
Multiple Sclerosis: Expert Review and Commentary from Published Literature
Cognitive Function as an Endpoint in Secondary Progressive Multiple Sclerosis
Sarah A. Morrow, MD, MS, FRCPC, FAAN
Associate Professor of Neurology, University of Western Ontario
Director, MS Clinic, London Health Sciences Centre
Founder, MS Cognitive Clinic, Parkwood Institute
London, Ontario
Most individuals with secondary progressive multiple sclerosis (SPMS) have or will develop measurable cognitive impairment. Reduced information processing speed and episodic memory loss are the most commonly affected domains. Cognitive impairment has been shown to be a source of disability and diminished quality of life that rivals physical symptoms. Although no therapy has been approved to prevent or reverse cognitive impairment associated with MS, there is recent evidence from a placebo-controlled therapeutic trial in SPMS showing a beneficial effect on cognitive impairment risk. In a secondary analysis of the phase 3 trial, those randomized to the active agent, a modulator of sphingosine-1-phosphate (S1P) receptor function, had a lower risk of cognitive impairment and a greater likelihood of improvement on measures of processing speed than those in the placebo arm. The trial provides a framework for considering how to evaluate and monitor cognitive function as a therapeutic target.
Background
Cognitive impairment is a well-documented consequence of the neurodegeneration associated with MS.1 The risk of cognitive impairment increases with disease progression, but it can occur independently of other symptoms. In a study of individuals with MRI findings suggestive of MS but who had no symptoms, cognitive impairment was identified in 27.6%.2 In these asymptomatic individuals, who met criteria for radiologically isolated syndrome (RIS), cognitive impairment correlated with higher T1 lesion volume and lower cortical volume.
For an exclusive interview with Dr. Sarah A. Morrow on the impact to clinical practice, click here
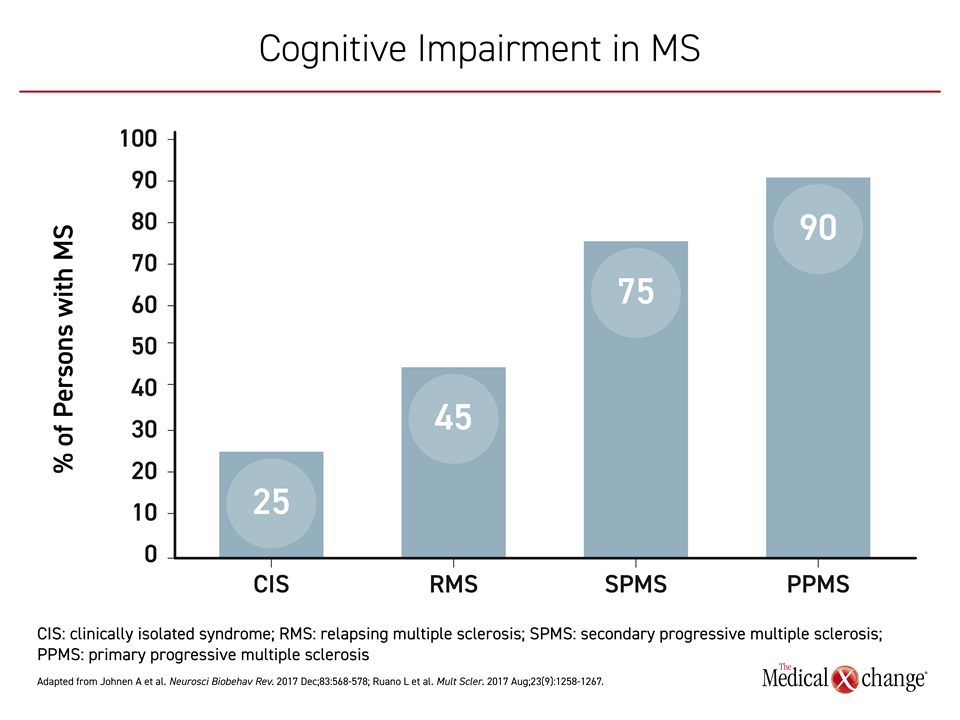
Cognitive impairment is already common noted in clinically isolated syndrome (CIS), reported in up to 25% of those with this condition,3 but the risk increases to 45% in those who progress to relapsing MS (RMS) and then as high as 75% in those with SPMS4 (Figure 1). Despite the stepwise increase in risk for cognitive impairment as MS progresses, the correlation is imperfect. Some persons with MS (PwMS) with no or little disability can exhibit substantial cognitive impairment. Conversely, cognitive impairment is not reported in other PwMS with advanced physical disability.5
Individuals who have stable MS without progression have been the source of additional evidence that physical disability and neurocognitive changes advance independently. Cognitive impairment has been observed in PwMS with very low disease activity, defined as an Expanded Disability Status Scale (EDSS) score of <3.0 for at least 15 years after diagnosis, even though cognitive impairment in these individuals is an adverse prognostic marker. When followed, those PwMS with low disease activity but cognitive impairment are more likely to worsen physically, as noted by an increase in EDSS when evaluated 5 or 12 years later.6,7 However, even this relationship is not absolute.
Similarly, pediatric onset of MS is associated with cognitive impairment, so that children and adolescents with MS on average have poorer academic performance and lower intelligence quotient scores than healthy controls.8 Again, this relationship is not absolute. When PwMS with a pediatric onset are followed longitudinally, cognitive symptoms and physical disability do not necessarily advance at the same pace over time.9
MS is an inflammatory and demyelinating disease of the central nervous system (CNS), making it reasonable to presume that cognitive impairment is a direct product of neurodegeneration.10 Yet, the exact pathophysiology is unclear. In a MRI study of 234 PwMS of which 41% had cognitive deficits, cortical grey matter volume loss was the only consistent baseline predictor of cognitive decline across MS subtypes, but predictors differed for those with early-stage RRMS, in whom white matter integrity damage predicted cognitive deficits, relative to those with late-stage RRMS or progressive MS, in whom cortical atrophy was a significant predictor.11 Greater cognitive decline was associated with greater structural damage at baseline in both groups, but one did not reliably predict the other in any individual patient.
Individual differences in brain reorganization may be relevant to the variability at which cognitive function advances at the level of an individual person. Brain reorganization refers to the process with which new neural pathways are formed to sustain functional connectivity in response to neurodegeneration. It has been hypothesized that some PwMS have greater capacity for brain reorganization.12 This theory is supported by several studies. In a functional connectivity analysis conducted in PwMS and controls, for example, those with MS demonstrated unequal activation of brain regions attributed to adaptive changes.13
There have been discordant findings regarding functional connectivity in the grey matter of brain structures. One explanation is that increased connectivity represents the capacity of the brain early in the disease to find alternative neural pathways to those damaged, while decreased connectivity represents a later stage where reserves have been exhausted, resulting in progressive cognitive deficit.1
Brain and cognitive reserve appear to be at least partially independent predictors of preserved cognitive function in PwMS. In a study that compared the effects of brain reserve, defined as maximal lifetime brain volume and presumed to be genetically determined, and cognitive reserve, which is derived from early-life leisure cognitive activities, the cognitive reserve was found to protect against cognitive decline independently of brain reserve.14 In a more recent study, cognitive and brain reserve were also found to have different roles with cognitive function more closely associated with classical cognitive domains and brain reserve more closely associated with social cognition.15 The variability in cognitive status across patients with similar patterns of MS as well as those with other diseases associated with cognitive loss, such as Alzheimer’s disease, is likely to be explained by these different types of neurologic reserve.16
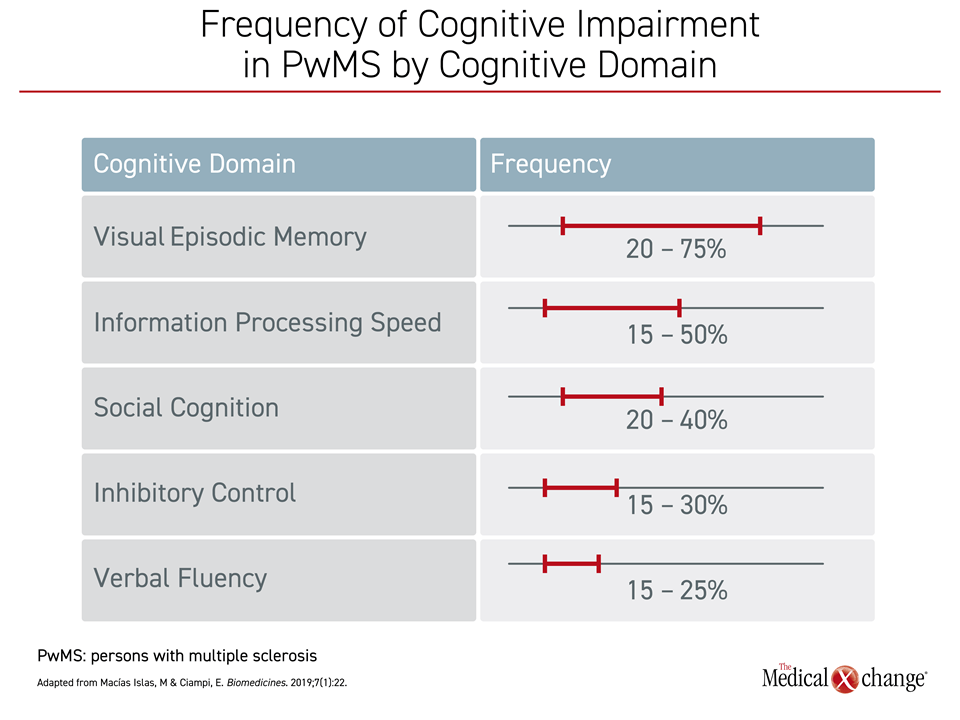
Despite some variability across studies, episodic memory loss and impaired information processing speed have been repeatedly identified as the most common affected domains in PwMS.17, 18 In a review that collated data from published studies evaluating impairment across domains of cognitive function, more than half of PwMS had impairment in one or both of these domains.19 In comparison, impaired verbal fluency was relatively uncommon (Figure 2).
The variability in cognitive impairment mirrors variability in physical symptoms, such as motor function, tremor, diplopia, or numbness. Although both are likely driven primarily by structural and functional changes related to neurodegeneration, the limited correlation in the speed and severity of progression suggests the pathways that drive each are, at least to some degree, independent. In a study attempting to determine whether structural or functional changes drive deficits in information processing speed, the correlation was found to be strongest for structural change. A more specific understanding of the pathophysiology is needed for its potential to lead to new strategies for treatment. A better understanding of modifiable drivers of cognitive impairment might also help to identify which of the existing disease-modifying treatments, if any, offer the greatest preservation of cognitive function.
It has been nearly 10 years since a multidisciplinary conference was assembled to advocate for the routine assessment and treatment of cognitive dysfunction in MS.20 When specific guidelines for managing cognitive decline were drafted in 2018, the authors emphasized that cognitive function, although a major source of morbidity, was still being inadequately evaluated or treated.21
However, clinical enthusiasm for monitoring this symptom is likely to have been tempered by the limited treatment options. Even in the 2018 guidelines, the review of potential pharmacologic therapies, such as amantadine, donepezil, and ginkgo biloba, did not identify any with convincing benefit. Rather, the most strongly advocated interventions were cognitive remediation and exercise. Both are supported by clinical studies,22, 23 but the therapeutic gain with each is modest.
Many clinicians recognize the rationale for establishing baseline cognitive function, but documenting change and the rate of change over time is also important.21 In children, this permits early intervention when symptoms threaten academic performance. In adults, the value ranges from early remediation to documenting disability for those who are candidates for benefits in the event of lost income. Tools other than SDMT can be considered, but guidelines emphasize serial reassessments with the same instrument. Along with cognitive screening, at least annual screening for depression, which can masquerade for or complicate cognitive impairment, is also recommended.
EXPAND Secondary Analysis: Impact on Cognitive Function
Despite the prominence of cognitive dysfunction as a symptom and source of disability in MS, few major treatment trials have included cognitive function among endpoints.21 In the phase 3 EXPAND trial, which compared siponimod to placebo, cognitive assessments were incorporated a priori as a study outcome. Assessments were performed at baseline, every six months, and at the end of treatment. The primary endpoints for this trial were time to three-month confirmed disability progression (CDP),24 while the cognitive outcomes were a prespecified exploratory analysis.
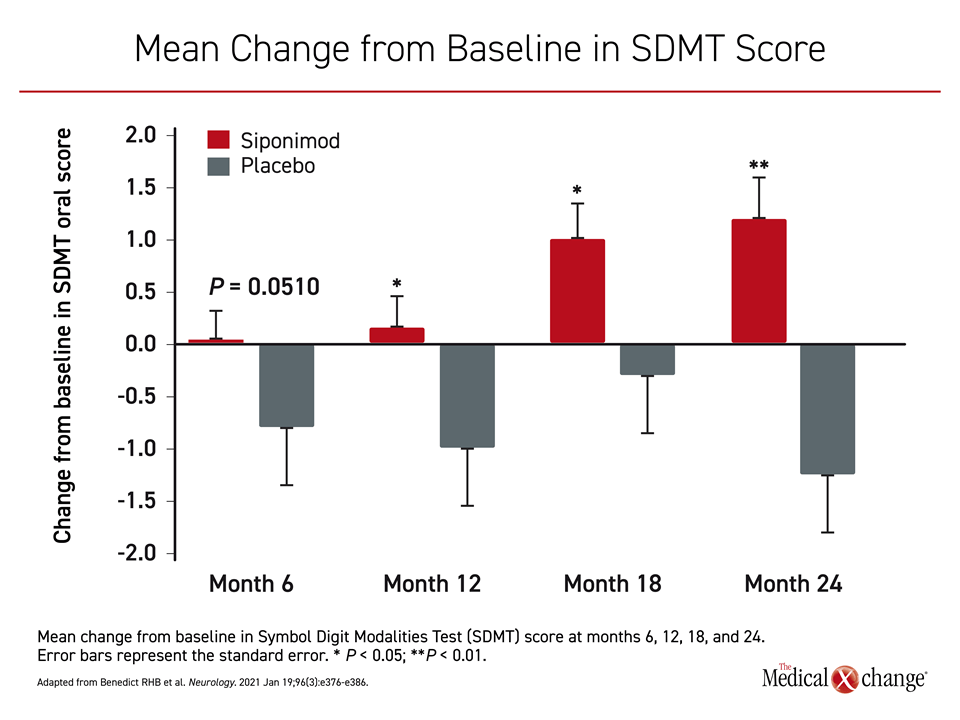
The results of the predefined and post hoc analysis of the cognitive outcomes were recently published.25 On the basis of the Symbol Digit Modalities Test (SDMT), which is the most commonly used and arguably the best measure of clinically meaningful change in information processing speed,26 there were significant differences favoring siponimod at month 12 (P=0.0132), month 18 (P=0.0135), and month 24 (P=0.0002) (Figure 3).
For a clinically meaningful change in SDMT, defined as a four-point change from baseline, those randomized to siponimod were at a significantly lower risk of decline in cognitive function with a hazard ratio (HR) of 0.79 (P=0.0157). The siponimod group also had a significantly greater likelihood of having improvement in SDMT scores (HR 1.28; P=0.0131). This improvement in cognitive function was observed in those with or without cognitive impairment prior to study entry, defined as a SDMT score of <43 points (higher scores signaling better performance) and in those with advanced disability, defined as EDSS score of 6 or greater, and in those with less disability. The authors considered the preservation of cognitive function consistent with MRI scans, which were performed every 12 months and associated siponimod with reduced brain volume loss relative to placebo.
In the core analysis of EXPAND, which randomized 1651 people with SPMS in a 2:1 ratio to 2 mg once daily of siponimod or placebo at centers in 31 participating countries, siponimod was associated with a 21% (P=0.013) reduction in the risk of reaching 3-month CDP. It was the first oral therapy to show a reduction in SPMS progression.
Of those who participated in the core study, 1224 were enrolled in an extension phase, when control subjects were switched to the active therapy. At the end of the extension study, when those originally randomized to siponimod were compared to those switched from placebo, an advantage was demonstrated for early initiation of the active therapy. This included a highly significant advantage for the primary endpoint of 3-month CDP (P=0.0064) and 6-month CDP (P=0.0048).27
In the cognitive outcome substudy, time to sustained changes in SDMT was evaluable in 98% of the study participants. Two additional cognitive assessments were administered. The Paced Auditory Serial Addition Test (PASAT) measures processing speed/working memory. The Brief Visuospatial Memory Test-Revised (BVMT-R) measures visual and spatial memory. In this study, both the Total Recall (TR) and Delayed Recall (DR) instruments of the BVMT-R were employed. No difference between active treatment and placebo groups was significant for any of these measures at 12- and 24-month assessments. This was not wholly unexpected. PASAT has been shown in prior studies to be more susceptible to practice-effects.28 BVMT-R is designed to test memory, for which functional improvements have never been achieved with a disease-modifying therapy in a phase 3 trial.
The improvement in SDMT is consistent with experimental studies associating siponimod with neuroprotection, including remyelination. In one ex vivo study, siponimod was associated with restoration of cortical neuronal circuit function.29 In a clinical study, siponimod has been associated with a reduction in cortical gray matter and thalamic volume loss.30 However, the exact mechanism by which siponimod favorably affected cognitive function in the EXPAND trial remains unclear.
According to the authors of the EXPAND secondary analysis on cognitive function, the improvement in SDMT score associated with siponimod would likely have a beneficial high impact on quality of life and vocational status.
Cognitive Impairment: Rationale for Monitoring and Treatment
By providing evidence that cognitive impairment can be prevented or reversed, the EXPAND trial establishes a new context for existing recommendations to screen and monitor PwMS for cognitive deficits. Baseline and serial cognitive assessments are already guideline- recommended, and the EXPAND trial shows the potential siponimod has for modifying cognitive decline in MS. It also emphasizes the need for openly discussing, measuring, and managing this manifestation of disease.
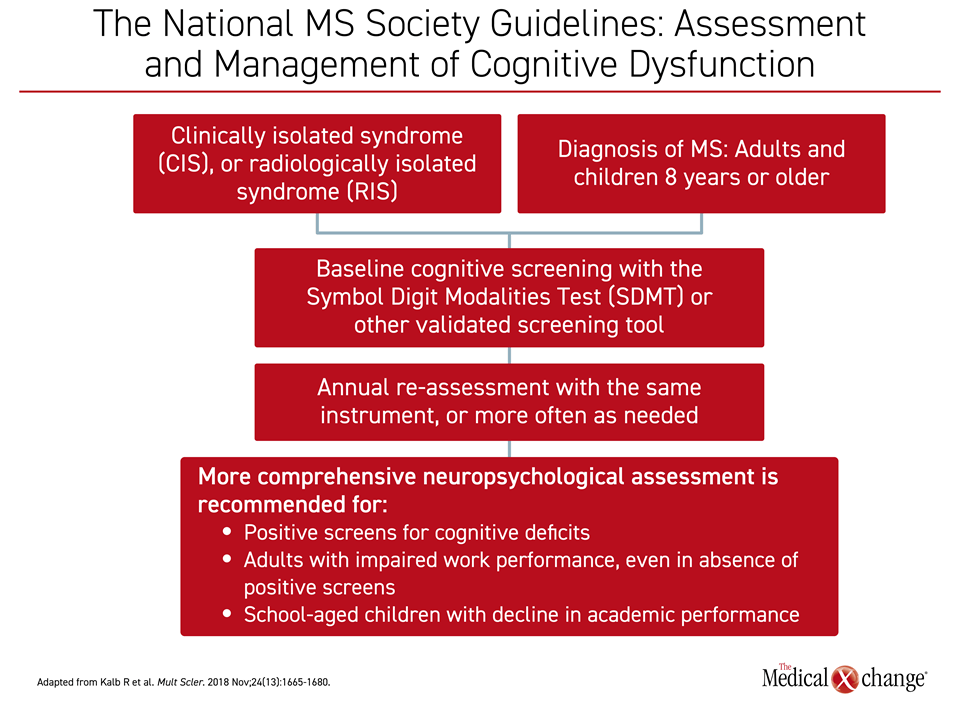
In the guidelines, cognitive screening with the SDMT or other validated screening tools is indicated in all adults and children 8 years old or older with CIS, RIS defined as early gray matter damage, or a diagnosis of MS. Reassessment in these individuals is recommended at yearly intervals or more often in the event of change in cognitive function (Figure 4). According to the guidelines, assessment and management of cognitive dysfunction should be raised to the same standards applied to prevention of relapses and progression of physical disabilities.
This is justified on the basis of evidence that burden imposed by cognitive impairment is of a magnitude at least equal to that of physical disabilities. In a study limited to those with a progressive form of MS, cognitive function assessments, including the SDMT, correlated closer with quality of life as measured with the Short-Form 36 (SF-36) than EDSS and other measures of physical impairment, such as the timed 25-foot walk (T25FW).31 In another study, which found a relationship between a decline in neuropsychological tests and adverse change in vocational status, a clinically significant decline in SDMT score raised the odds ratio of vocational status deterioration by more than four-fold.32
In a more complex study that constructed a model to evaluate predictors of work disability, cognitive impairment as measured with SDMT, physical disability as measured with EDSS, and depression evaluated with the Hospital Anxiety and Depression Scale (HADS-D) were all significant (P<0.05).33 All were identified as potential targets for preserving or improving vocational status.
People with MS who are cognitively impaired score lower on measures of quality of life than those who have similar physical disabilities but no cognitive impairment.34 Greater cognitive impairment imposes greater restrictions on daily activities, such as driving a car or participating in social activities.35, 36 Slow information processing is associated with lower income in PwMS independent of physical dysfunction.37
According to the 2018 guidelines, evaluation and management of cognitive impairment in MS is as important as evaluation and management of physical symptoms. This is relevant to the earliest stages of MS but grows more urgent with progressive disease when cognitive impairment is more common and may be more severe. The failure of clinicians to address cognitive impairment with the same rigor and energy that is applied to other symptoms was identified as a major barrier to optimal care.
Conclusion
A secondary analysis of the pivotal EXPAND trial provides the best evidence so far that an active therapy in SPMS can preserve and, in some cases, improve cognitive function. This class II evidence draws attention to an important aspect of MS that is sometimes overlooked. Insufficient appreciation of the relative adverse impact exerted by cognitive impairment on quality of life, insufficient knowledge and training on how to evaluate and monitor cognitive function, and lack of awareness about strategies to work with PwMS to modify cognitive impairment have all been barriers to optimal management of this MS symptom.21 Treatments to prevent or reverse cognitive impairments are needed urgently.
References
1. Benedict RHB, Amato MP, Deluca J, et al. Cognitive impairment in multiple sclerosis: clinical management, MRI, and therapeutic avenues. Lancet Neurol. 2020;19:860-871.
2. Amato MP, Hakiki B, Goretti B, et al. Association of MRI metrics and cognitive impairment in radiologically isolated syndromes. Neurology. 2012;78:309-314,
3. Feuilet F, Reuter F, Audoin B, et al. Early cognitive impairment in patients with clinically isolated syndrome suggestive of multiple sclerosis. Mult Scler. 2007;13:124-127.
4. Johnen A, Landmeyer N, Burkner PC, et al. Distinct cognitive impairments in different disease courses of multiple sclerosis: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2017;83:568-578.
5. Ruano L, Portaccio E, Goretti B, et al. Age and disability drive cognitive impairment in multiple sclerosis across disease subtypes. Mult Scler. 2017;23:1258-1267.
6. Portaccio E, Stromillo ML, Goretti B, et al. Neuropsychological and MRI measures predict short-term evolution in benign multiple sclerosis. Neurology. 2009;73:498-503.
7. Razzolini L Portaccio E, Stromillo ML, et al. The dilemma of benign multiple sclerosis: can we predict the risk of losing the “benign status”? A 12-year follow-up study. Mult Scler Relat Disord. 2018;26:71-73.
8. Charvet LE, O’Donnell EH, Belman AL, et al. Longitudinal evaluation of cognitive functioning in pediatric multiple sclerosis: report from the US Pediatric Multiple Sclerosis Network. Mult Scler .2014;20:1502-1510.
9. WaldmanA, Ness J, Pohl D, et al. Pediatric multiple sclerosis: clinical features and outcome. Neurology. 2016;87(suppl 2):S74-S81.
10. Benedict RHB, Amato MP, DeLuca J, et al. Cognitive impairment in multiple sclerosis: clinical management, MRI, and therapeutic avenues. Lancet Neurol. 2020;19:860-871.
11. Eijlers AJC, van Geest Q, Dekker I, et al. Predicting cognitive decline in multiple sclerosis: a 5-year follow-up study. Brain. 2018;141:2605-2618.
12. Nasios G, Bakirtzis C, Messinis L. Cognitive impairment and brain reorganization in MS: underlying mechanisms and the role of neurorehabilitation. Front Neurol. 2020;11:147.
13. Cader S, Cifelli A, Abu-Omar Y, et al. Reduced brain functional reserve and altered functional connectivity in patients with multiple sclerosis. Brain. 2006;129(Pt 2):527-537.
14. Sumowski JF, Rocca MA, Leavitt VM, et al. Brain reserve and cognitive reserve in multiple sclerosis; what you’ve got and how you use it. Neurology. 2013;80:2186-2193.
15. Machado R, Lima C, d’Almeida OC, et al. Protective effects of cognitive and brain reserve in multiple sclerosis: differential roles on social cognition and ‘classic cognition.’ Mult Scler Relat Disord. 2021;48:102716.
16. Sumowski JF. Cognitive reserve as a useful concept for early intervention research in multiple sclerosis. Front Neurol. 2015;6:176.
17. Chiaravalloti MD, DeLuca J. Cognitive impairment in multiple sclerosis. 2008;7:1139-1151.
18. Langdon DW. Cognition in multiple sclerosis. Curr Opin Neurol .2011;24:244-249.
19. Islas MAM, Ciampi E. Assessment and impact of cognitive impairment in multiple sclerosis: an overview. Biomedicines. 2019;7:22.
20. Foley FW, Benedict RHB, Gromisch ES, et al. The need for screening, assessment, and treatment for cognitive function in multiple sclerosis: results of a multidisciplinary CMSC consensus conference, September 24, 2010. Int J MS Care. 2012;14:58-64.
21. Kalb R, Beier M, Benedict RHB, et al. Recommendations for cognitive screening and management in multiple sclerosis care. Mult Scler J. 2018;24:1665-1680.
22. Charvet LE, Yang J, Shaw MT, et al. Cognitive function in multiple sclerosis improves with telerehabilitation: results from a randomized controlled trial. PLoS One. 2017;12:e0177177.
23. Motl RW, Sandroff BM, DeLuca J. Exercise training and cognitive rehabilitation: a symbiotic approach for rehabilitating walking and cognitive functions in multiple sclerosis? Neurorehabil Neural Repair. 2016;30:499-511.
24. Kappos L, Bar-Or A, Cree BAC, et al. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomized, phase 3 study. Lancet Neurol. 2018;391:1263-1273.
25. Benedict RHB, Tomic D, Cree BA, et al. Siponimod and cognition in secondary progressive multiple sclerosis: EXPAND secondary analyses. Neurology. 2021;96:e376-e386.
26. Benedict RHB, DeLuca J, Phillips G, et al. Validity of the Symbol Digit Modalities Test as a cognition performance outcomes measure for multiple sclerosis. Mult Scler. 2017;23:721-733.
27. Kappos L, Cree BCC, Benedict RHB, et al. Long-term efficacy and safety of siponimod in patients with SPMS: EXPAND extension analysis up to 5 years. Neurology. 2020;94(15 suppl):4128.
28. Barker-Collo SL. Within session practice effects on the PASAT in clients with multiple sclerosis. Arch Clin Neuropsychol. 2005;20:145-152.
29. Hundehege P, Cerina M, Eichler S, et al. The next-generation sphingosine-1 receptor modulator BAF312 (siponimod) improves cortical network functionality in focal autoimmune encephalomyelitis. Neural Regen Res. 2019;14:1950-1960.
30. Arnold DA, Fox R, Bar-Or A, et al. Effect of siponimod on cortical grey matter and thalamic volume in patients with secondary progressive multiple sclerosis: results of the EXPAND study. Presented at 25th Congress of the European Committee for Treatment and Research in Multiple Sclerosis. Stockholm 2019. Poster P382.
31. Chow HH, Schreiber K, Magyari M, et al. Progressive multiple sclerosis, cognitive function, and quality of life. Brain Behav. 2018;8:e00875.
32. Morrow SA, Drake A, Zivadinov R, et al. Predicting loss of employment over three years in multiple sclerosis: clinically meaningful cognitive decline. Clin Neuropsychol 2010;24:1131-1145.
33. Povolo CA, Blair M, Mehta S, et al. Predictors of vocational status among persons with multiple sclerosis. Mult Scler Relat Disord. 2019;36:101411.
34. Campbell J, Rashid W, Cercignani M, et al. Cognitive impairment among patients with multiple sclerosis: associations with employment and quality of life. Postgrad Med J. 2017;93:143-147.
35. Morrow SA, Classen S, Monahan M, et al. On-road assessment of fitness-to-drive in persons with MS with cognitive impairment: a prospective study. Mult Scler. 2018;24:1499-1506.
36. Cattaneo D, Lamers I, Bertoni R, et al. Participation restriction in people with multiple sclerosis: prevalence and correlations with cognitive, walking, balance, and upper limb impairments. Arch Phys Rehabil. 2017;98:1308-1315.
37. Kavaliunas A, Tinghog P, Friberg E, et al. Cognitive function predicts work disability among multiple sclerosis patients. Mult Scler J Exp Transl Clin. 2019;5:20552173188221234.