Cardiology
Heart Failure: Expert Review and Commentary from Published Literature
New Data Emphasize Prompt Initiation of ARNI Therapy as a Standard of Care for HFrEF in Patients With and Without Diabetes
Kim Connelly, MBBS PhD, FSCMR, FCCS
Associate Professor of Medicine, University of Toronto
Toronto, Ontario
Michel White MD, FRCP(C), FACC, FESC
Professor of Medicine, Université de Montréal
Carolyn and Richard Renaud Research Chair in Heart Failure of the Montreal Heart Institute
Montreal, Quebec
Each of four therapies now regarded as the pillars of treatment for heart failure with reduced ejection fraction (HFrEF) have demonstrated a survival benefit. When it was declared a core pillar, the angiotensin receptor neprilysin inhibitor (ARNI) sacubitril/valsartan had demonstrated a reduction in all-cause mortality when added to the previous standards of care in the landmark PARADIGM-HF trial. The benefit is largely attributed to down regulation of neurohormonal activation, and decrease in N-terminal pro-B type natriuretic peptide (NT-proBNP).1 In a subsequent study, PROVE-HF, this effect at 12 months was associated with reversal of the cardiac remodelling that characterizes HFrEF as well as a nearly 10% improvement in left ventricular ejection fraction (LVEF) (P<0.001).2 In a subsequent and newly-published analysis from this same study, a comparable magnitude of effect was confirmed in those with type 2 diabetes mellitus (T2DM).3 This and other evidence that an ARNI reverses not just slows the underlying pathology of HFrEF is the reason new guidelines recommend prompt initiation of this therapy, like the other pillars, in HFrEF in patients with or without T2DM.
Background
All four pillars of contemporary guideline-directed medical therapy (GDMT) for HFrEF have been associated with a survival benefit. In each case, the landmark studies that established their efficacy showed this survival benefit in patients already on optimal GDMT. After it demonstrated a 20% reduction (P<0.001) in the composite primary endpoint of major heart failure events relative to the ACE inhibitor enalapril in the PARADIGM-HF trial, the ARNI sacubitril/valsartan joined beta blockers and mineralocorticoid antagonists (MRA) as a third pillar of standard treatment in HFrEF, supplanting ACE inhibitors, in 2017 guidelines from the Canadian Cardiovascular Society (CCS).4 In addition to the reduction in these events—cardiovascular death and hospitalization for heart failure—the ARNI provided a 16% reduction (P<0.001) in risk of all-cause mortality. Most of the patients in the PARADIGM-HF trial were on a combination of treatments considered to be the standard at the time, including beta blockers in 90% and a MRA in more than half.
For an exclusive interview with Dr. Kim Connelly on the impact to clinical practice, click here
A fourth pillar of HFrEF treatment was subsequently added on the basis of the DAPA-HF and EMPEROR-Reduced trials. In the DAPA-HF trial, the sodium-glucose co-transporter 2 (SGLT2) inhibitor dapagliflozin was associated with a 26% reduction (P<0.001) in a composite heart failure outcome relative to placebo.5 The protection from these events—cardiovascular death and urgent intravenous treatment for heart failure— was accompanied by a 17% reduction (P<0.001) in all-cause mortality. In the EMPEROR-Reduced trial, the SGLT2 inhibitor dapagliflozin was associated with a 25% reduction (P<0.001) relative to placebo in a similar composite heart failure endpoint.6
In the SGLT2 trials, like PARADIGM-HF with sacubitril-valsartan, the benefit was similar in those with or without T2DM. In all three trials, the benefits were achieved on top of the previous standard for HFrEF treatment. Although an ARNI was not yet a standard when the DAPA-HF and EMPEROR-Reduced trials were initiated, 10% of those in DAPA-HF and 20% of those in EMPEROR-Reduced were on this therapy, while more than 90% were taking beta blockers, more than 70% were on an MRA, and nearly all of those not on ARNI were taking a renin-angiotensin inhibitor.
To fully appreciate the concept of the four pillars of GDMT, it is essential to grasp that these mortality reductions are additive.7 While the CCS first updated its guidelines last year to include SGLT2 inhibitors in HFrEF patients with or without diabetes mellitus,5 they have now been superseded by a more recent and more comprehensive update. The most recent update more explicitly identifies beta blockers, MRA, ARNI, and SGLT2 inhibitors as the four key therapeutic drug classes as first-line therapy that should be offered to all patients without a contraindication.8 Moreover, these guidelines recommend starting all of these therapies in a timely manner to achieve optimal clinical benefits.
The American College of Cardiology (ACC), which recently has updated its decision pathway for HFrEF, has made the same recommendation.9 It defined standard first-line GDMT for HFrEF as beta blockers, MRAs, ARNI, and SGLT2 inhibitors. According to the ACC update, these should be considered in all HFrEF populations irrespective of age or concomitant T2DM. Recently, the estimated benefit of employing all four therapies was up to 8.3 additional years of life free of major HFrEF-related events in a 55 year old and up to 2.7 years in an 80-year old.10
The reason that all four GDMT therapies should be employed together and promptly after a diagnosis of HFrEF is that their actions are complementary and additive. There is no standard sequence for initiation of GDMT, but the updated ACC pathway advised that starting with a beta blocker or an ARNI is reasonable. However, these treatments are not hierarchical. Different strategies for efficiently bringing patients up to optimal doses for all four agents will be influenced by patient-specific considerations, such as treatments the patient is already taking, but all deserve recognition as first-line treatments.
The rationale for placing particular stress on the value of prompt introduction of the ARNI is persistent evidence of a care gap specific to this agent. Slow adoption of new guidelines is a common issue in medicine, but it appears that a large segment of HFrEF patients are not receiving a therapy with a documented survival benefit. In a survey of more than 200,000 HFrEF patients in Canada who met eligibility criteria for ARNI in 2018, less than 15% were on this therapy.11 This major gap in care is inconsistent with guidelines and the evidence. Like the other four pillars of HFrEF treatment, ARNI acts on a major driver of HFrEF pathology, but ARNI has the additional advantage of being associated with reverse cardiac remodeling.
ARNI: Targeting Fundamental Pathology in HFrEF
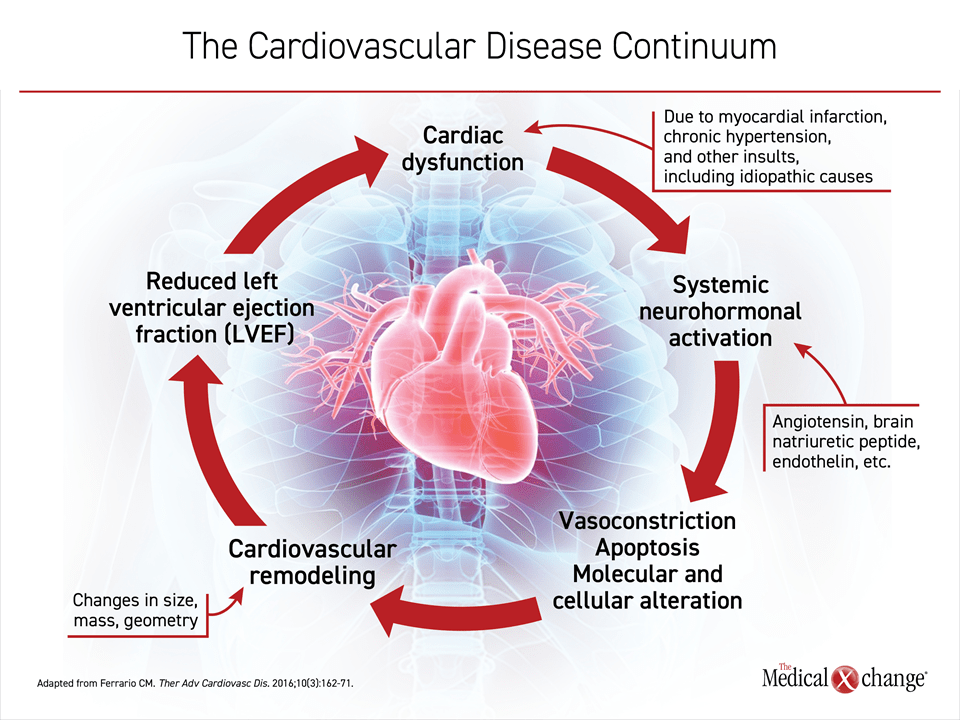
The PARADIGM-HF trial associated ARNI with a major clinical benefit. Subsequent studies have provided additional information about the mechanism. While the steep reductions in NT-proBNP achieved with ARNI were assumed to the basis for a slowing of disease progression, the PROVE-HF study correlated the reductions in NT-proBNP with reverse cardiac remodeling.2 This suggests a fundamental benefit on disease pathophysiology. The value of reversing not just slowing cardiac remodeling is best understood within the cardiovascular continuum of HFrEF, characterized nearly 20 years ago.12 This continuum describes a vicious cycle of progressive and ultimately terminal deterioration of heart function that begins with neurohormonal activation (Figure 1).
It is now apparent that the suppression of activated neurohormones, including those of the renin-angiotensin-aldosterone system (RAAS) and NT-proBNP, modulate HFrEF progression.13 Circulating levels of these neurohormones rise rapidly in response to a myocardial infarction (MI) but are also activated after more slowly evolving insults such as hyperglycemia.14 Acutely, the activation of neurohormones mediate steps that favor early cardiovascular homeostasis, such as vasoconstriction and water retention. However, chronic neurohormone activation ultimately produces deleterious changes in cardiac structure, such as ventricular wall remodeling, that impair cardiac function and initiate the vicious cycle of that underlies HFrEF progression.13
All four of the HFrEF pillar therapies derive at least part of their clinical benefit from their ability to diminish the deleterious effects of neurohormone activation. In the case of ARNI, the benefit is provided by the neprilysin inhibitor sacubitril, which prevents the degradation of NT-proBNP. The impact of neprilysin inhibition, combined with RAS blockade from the valsartan component of the ARNI, is powerful ventricular unloading, which improves cardiac function secondary to a reduction in neurohormonal activation. This is demonstrated by the sustained reduction in NT-proBNP.15 The valsartan component prevents potential counterproductive upregulation of angiotensin by neprilysin inhibition while targeting another hormone participating in HFrEF pathophysiology. PARADIGM-HF demonstrated that the combined inhibition of neprilysin and valsartan improved HFrEF outcomes, but subsequent studies, including PROVE-HF, reverses the cardiac remodeling that underlies HFrEF.2
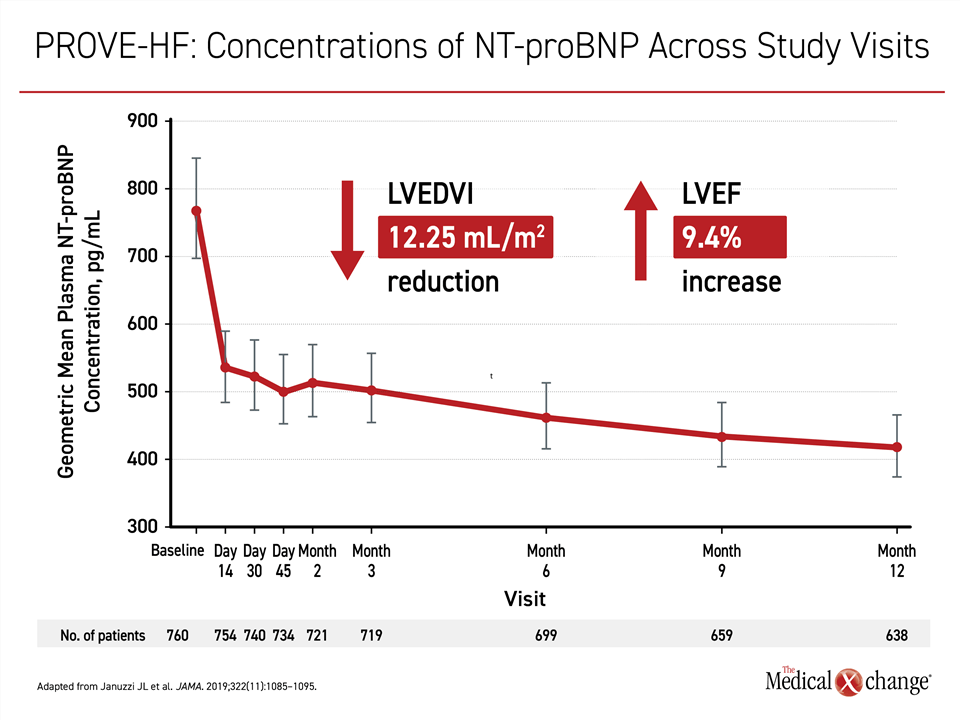
In the prospective PROVE-HF trial, circulating levels of NT-proBNP were monitored along with several measures of cardiac structure on echocardiography over a 12-month period in 794 HFrEF patients who initiated therapy with sacubitril-valsartan. Echocardiographic measures of left ventricular ejection fraction (LVEF) were assessed for changes in cardiac function.
From a baseline of 816 pg/mL, the median NT-proBNP concentration fell rapidly, reaching a median of 455 pg/mL, which represented a 45% reduction. Importantly, reductions in NT-proBNP correlated with favorable echocardiographic changes involving left ventricular end-diastolic volume index, (LVEDVI), left ventricular end-systolic volume index (LVESVI), left atrial volume index, and the ratio of early diastolic filling/early diastolic annular velocity (all P<0.001) (Figure 2). Reversed cardiac remodeling was detectable at six months, but more pronounced at 12 months.
These changes were accompanied by improvements in cardiac function as measured with LVEF. By the end of 12 months, median LVEF increased by almost 10%, rising from 28.2% to 37.8% (P<0.001). The improvement from baseline in structural improvements and the increase in LVEF associated with ARNI were all achieved on top of a background therapy that included beta blockers in 95% of patients and an MRA in approximately one third of patients.
The correlation between the reductions in NT-proBNP and the evidence of reverse cardiac remodeling were consistent across multiple stratifications. It was also observed in patient groups who had been excluded from the PARADIGM-HF study, such as those with modest elevations in natriuretic peptide concentrations or those who were not on stable doses of an angiotensin system inhibitor at the start of treatment.
Significance of ARNI Efficacy in T2DM
More recently, a post-hoc analysis of PROVE-HF demonstrated that the benefit of ARNI was comparable in patients with T2DM.3 Elevated HbA1c is associated with increased levels of circulating NT-proBNP and both are independently associated with increased cardiac mortality.16 Of patients with HFrEF, those with T2DM are a particularly important target of optimal treatment. The prevalence of heart failure in T2DM is approximately four times greater than that of the general population,17 while approximately 40% of patients with heart failure have diabetes.18 Ultimately, the rapidly rising rates of HFrEF in Canada and elsewhere can be traced to the ongoing epidemic of T2DM.19
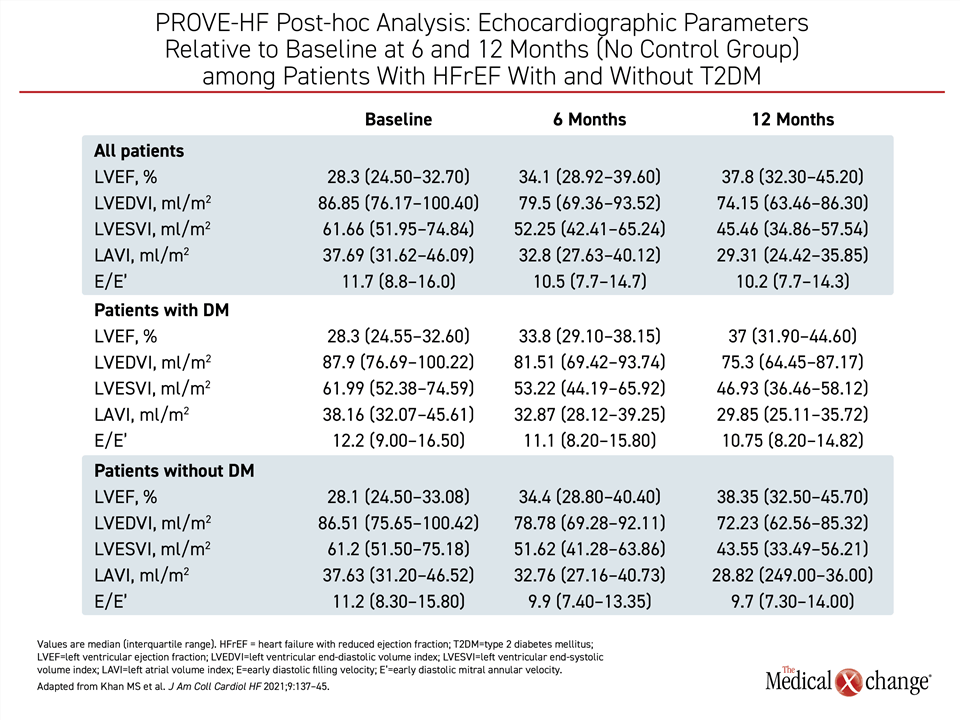
Of the 794 patients in the PROVE-HF study, 361 (45.5%) had T2DM. T2DM patients were less likely to have a history of MI (39.3% vs. 44.0%; P=0.02). A high proportion of the T2DM patients, like those without T2DM, entered the study on optimized therapy, which included beta blockers in more than 95% of patients and an angiotensin system inhibitor in more than 75%.
The T2DM subgroup entered PROVE-HF with a higher mean level of NT-proBNP (854.1 vs. 706.3 pg/mL), but the steep NT-proBNP reductions in this group paralleled those seen in non-diabetics. This included a 39% reduction in NT-proBNP by day 14 and a 43% reduction by end of study. Over 12 months of follow-up, the improvements in echocardiographic structural parameters, including LVEDVI, LVESVI, and LAVI, were similar in those with or without T2DM (Table 1).
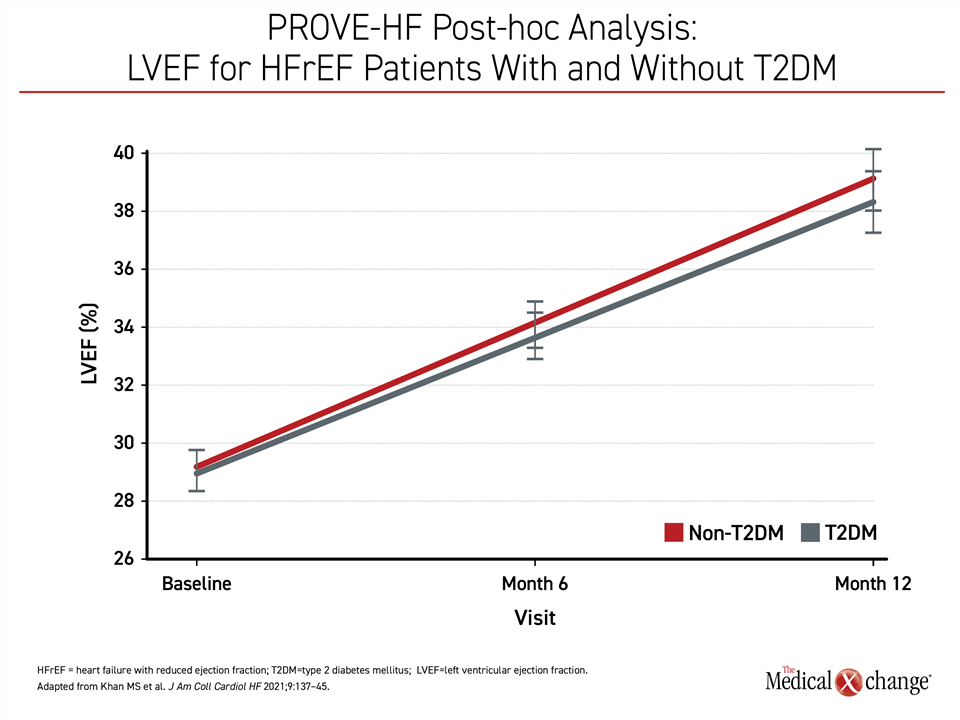
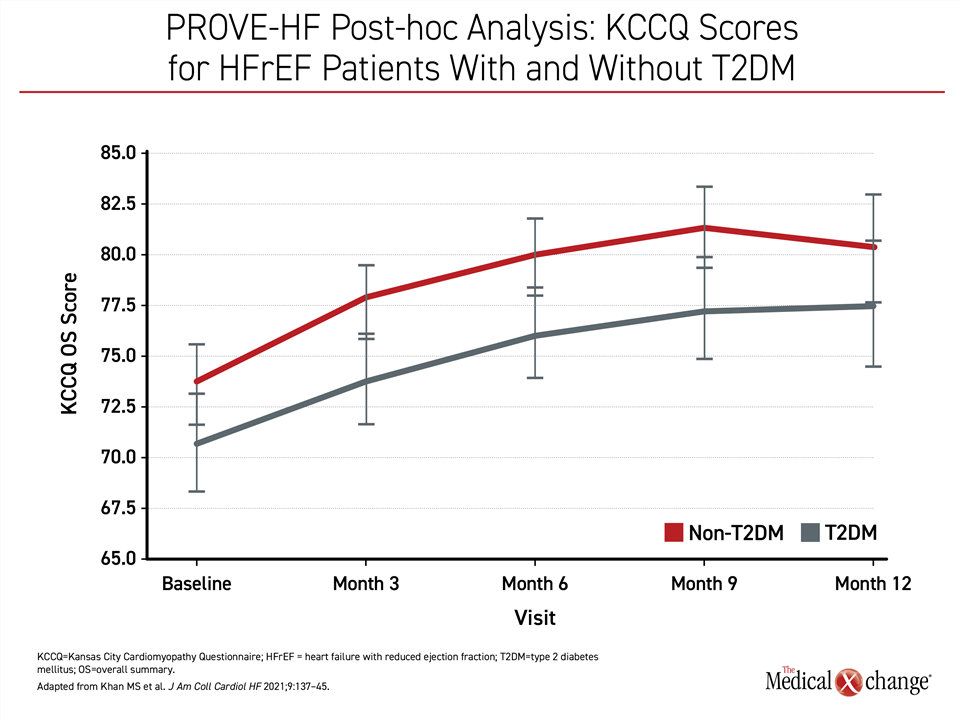
The end-of-study improvement in heart function as measured with LVEF also mirrored that observed in the non-T2DM group, rising from a baseline of 28.3% to 37%, or a gain of just under 9% (Figure 3).
The gains in cardiac function were clinically significant whether or not patients had T2DM, according to quality-of-life (QOL) assessed at 2, 3, 4, 6, 7, 8, 9, and 12 months with the Kansas City Cardiomyopathy Questionnaire (KCCQ)-23. In patients with T2DM relative to those without, baseline overall KCCQ scores (60.4 vs. 66.8) and total symptom scores (70.8 vs. 76,0) were slightly lower, signifying worse QOL, in those with T2DM, but the absolute gains for those with T2DM were slightly greater at both six months and 12 months. A five-point gain in the KCCQ is considered clinically significant,20 so the improvement in both groups was robust. Over 12 months, improvement in symptom score domains was observed in both groups. Although the score remained lower in the T2DM group (83.3 vs. 87.5) the gap had narrowed, suggesting the benefit was at least as good in this subpopulation (Figure 4).
The recent addition of SGLT2 inhibitors as a pillar of treatment in HFrEF should not overshadow the role of ARNI as one of the four fundamental pharmacologic treatments in this important subgroup. In PROVE-HF post-hoc analysis, the reverse cardiac remodeling and improvement in cardiac function was of a similar magnitude in those with T2DM as in those without. In T2DM patients with HFrEF, the risk of all of the major adverse outcomes, including a 50% greater likelihood of HFrEF-related hospitalizations, are substantially increased.19 As a component of GDMT, ARNI is at least as important in HFrEF patients with T2DM as those without.
ARNI Is a First Line GDMT in HFrEF
At the time the PARADIGM-HF trial was conducted, sacubitril/valsartan was an experimental drug, prompting a cautious titration scheme to ensure safety. Even though there was a low frequency of significant adverse events in those randomized to ARNI relative to enalapril, including symptomatic hypotension (2.7% vs. 1.4%), initial treatment guidelines, reflecting the PARADIGM-HF trial design, outlined a cautious switch to ARNI from an angiotensin system inhibitor. The language, including a recommendation for a washout period when switching from an ACE inhibitor to ARNI, might be responsible for the misperception, particularly among non-specialists, that there is less urgency initiating ARNI relative to the other HFrEF treatment pillars.
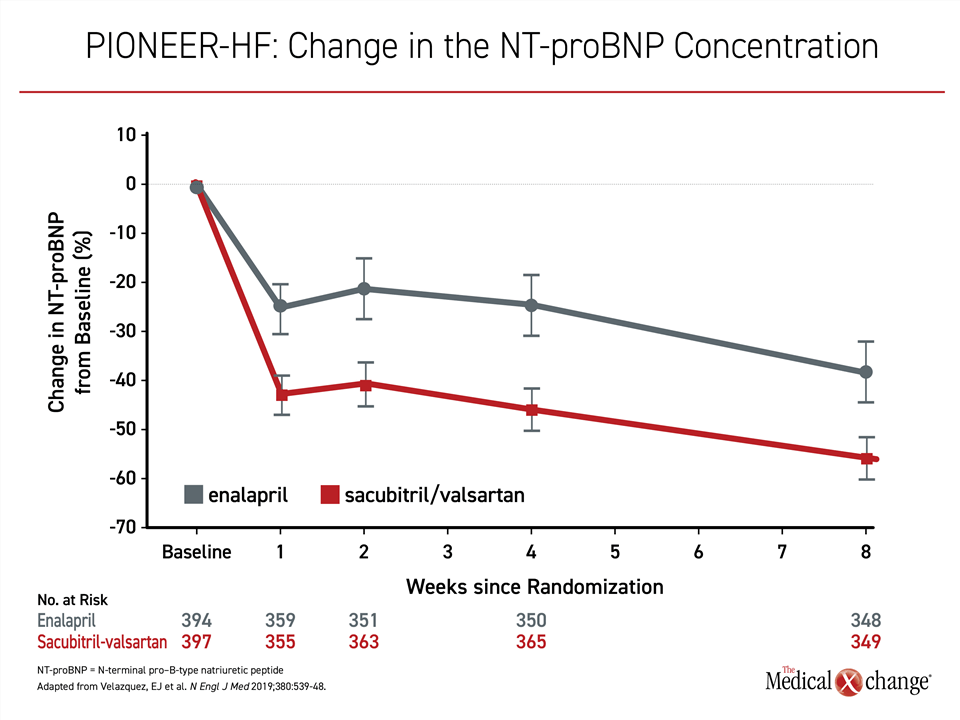
Wider experience with sacubitril/valsartan has expanded the body of evidence that this agent can be safely initiated during the index hospitalization of HFrEF. When initiated rapidly, including during hospitalization, the PIONEER-HF trial demonstrated a reduction in NT-proBNP favoring ARNI therapy over enalapril within the first week21 (Figure 5). Although the nearly 50% reduction in rehospitalization for heart failure and >30% reduction in death observed in the sacubitril/valsartan group in the relatively short eight weeks of follow-up in PIONEER-HF did not reach significance, the ability to rapidly reduce NT-proBNP with acceptable safety has provided the basis for current recommendations for early administration, including at the time of first hospitalization.
Other data collected since completion of the PARADIGM-HF study also reinforce the efficacy and safety of ARNI treatment. In a meta-analysis of 27 randomized and controlled trials, ARNI was associated with a mortality reduction of 14% (HR 0.86; 95% CI 0.79 – 0.94).22 Serious adverse events, including hypokalemia and angioedema, were lower in the ARNI-treated patients (HR 0.89, 95% CI 0.86 – 0.93).
In HFrEF patients not previously exposed to an angiotensin system inhibitor, initiating therapy with an ARNI is safe and effective.23,24 A direct-to-ARNI approach was recommended in the updated ACC pathway.9 Although prudent upwards titration of this drug, like other GDMT pillars of HFrEF, is advocated in the updated ACC pathway, the authors outlined specific strategies to circumvent obstacles to reaching and sustaining target doses of all four pillars, including ARNI. This included strategies to help patients with adherence, costs of treatment, and other practical challenges to optimal care.
For primary care physicians or other non-specialists managing HFrEF, specialist referrals might be appropriate in a number of patient groups, such as those with arrhythmias, persistent symptoms unresponsive to standard therapies, and need for chronic intravenous inotropes. However, assistance in achieving optimal dosing of GDMT is also a justification for referral.
In the landmark trials that provided the basis for the third and fourth pillars of treatment, the proportion of patients on standard-of-care therapies at baseline were reassuring. ACE inhibitors, which ARNI has now superseded, and beta blockers have provided a foundation for HFrEF for almost 30 years. The evidence of benefit from MRA soon followed. Although ARNI and SGLT2 inhibitors are newer additions, they are not adjunctive. Rather, these treatments, like the previous standards, should be offered routinely to every patient without a contraindication and initiated as quickly as possible after the diagnosis has been made. In the PARADIGM-HF trial, for example, divergence in the curves for the primary outcomes was visible within the first year or treatment.25 Optimal risk reductions from the four GDMT pillars of treatment in HFrEF depend on concomitant use.
Conclusion
The four pillars of GDMT HFrEF therapy are evidence-based. Pivotal trials have associated each with a reduction in HFrEF-related hospitalizations and improved survival. Patients at highest risk for poor outcomes, such as those with T2DM, will benefit most. The 2021 CCS guidelines for HFrEF defined concomitant treatment with all four therapies as a first-line standard. The optimal order with which to introduce these therapies might not be the same for all patients, but the goal should be to get HFrEF patients to target doses of all four agents promptly. It is the added value of these therapies to slow or even reverse disease progression, prevent HFrEF-related hospitalizations, improve quality of life, and extend survival that makes concomitant treatment the definition of optimal care.
References
1. McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371(11):993-1004. DOI: 10.1056/NEJMoa1409077.
2. Januzzi JL, Jr., Prescott MF, Butler J, et al. Association of Change in N-Terminal Pro-B-Type Natriuretic Peptide Following Initiation of Sacubitril-Valsartan Treatment With Cardiac Structure and Function in Patients With Heart Failure With Reduced Ejection Fraction. JAMA 2019:1-11. DOI: 10.1001/jama.2019.12821.
3. Khan MS, Felker GM, Pina IL, et al. Reverse Cardiac Remodeling Following Initiation of Sacubitril/Valsartan in Patients With Heart Failure With and Without Diabetes. JACC Heart Fail 2021;9(2):137-145. DOI: 10.1016/j.jchf.2020.09.014.
4. Ezekowitz JA, O’Meara E, McDonald MA, et al. 2017 Comprehensive Update of the Canadian Cardiovascular Society Guidelines for the Management of Heart Failure. Can J Cardiol 2017;33(11):1342-1433. DOI: 10.1016/j.cjca.2017.08.022.
5. O’Meara E, McDonald M, Chan M, et al. CCS/CHFS Heart Failure Guidelines: Clinical Trial Update on Functional Mitral Regurgitation, SGLT2 Inhibitors, ARNI in HFpEF, and Tafamidis in Amyloidosis. Can J Cardiol 2020;36(2):159-169. DOI: 10.1016/j.cjca.2019.11.036.
6. Packer M, Anker SD, Butler J, et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N Engl J Med 2020;383(15):1413-1424. DOI: 10.1056/NEJMoa2022190.
7. Burnett H, Earley A, Voors AA, et al. Thirty Years of Evidence on the Efficacy of Drug Treatments for Chronic Heart Failure With Reduced Ejection Fraction: A Network Meta-Analysis. Circ Heart Fail 2017;10(1). DOI: 10.1161/CIRCHEARTFAILURE.116.003529.
8. McDonald M, Virani S, Chan M, Ducharme A. CCS/CHFS heart failure guidelines update: defining a new pharmacologic standard of care for heart failure with reduced ejection fraction. Can J Cardiol 2021;37:531-546.
9. Writing C, Maddox TM, Januzzi JL, Jr., et al. 2021 Update to the 2017 ACC Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment: Answers to 10 Pivotal Issues About Heart Failure With Reduced Ejection Fraction: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol 2021;77(6):772-810. DOI: 10.1016/j.jacc.2020.11.022.
10. Vaduganathan M, Claggett BL, Jhund PS, et al. Estimating lifetime benefits of comprehensive disease-modifying pharmacological therapies in patients with heart failure with reduced ejection fraction: a comparative analysis of three randomised controlled trials. Lancet 2020;396(10244):121-128. DOI: 10.1016/S0140-6736(20)30748-0.
11. Huitema AA, Daoust A, Anderson K, et al. Optimal Usage of Sacubitril/Valsartan for the Treatment of Heart Failure: The Importance of Optimizing Heart Failure Care in Canada. CJC Open 2020;2(5):321-327. DOI: 10.1016/j.cjco.2020.03.015.
12. Dzau VJ, Antman EM, Black HR, et al. The cardiovascular disease continuum validated: clinical evidence of improved patient outcomes: part I: Pathophysiology and clinical trial evidence (risk factors through stable coronary artery disease). Circulation 2006;114(25):2850-70. DOI: 10.1161/CIRCULATIONAHA.106.655688.
13. Hartupee J, Mann DL. Neurohormonal activation in heart failure with reduced ejection fraction. Nat Rev Cardiol 2017;14(1):30-38. DOI: 10.1038/nrcardio.2016.163.
14. Magnusson M, Melander O, Israelsson B, Grubb A, Groop L, Jovinge S. Elevated plasma levels of Nt-proBNP in patients with type 2 diabetes without overt cardiovascular disease. Diabetes Care 2004;27(8):1929-35. DOI: 10.2337/diacare.27.8.1929.
15. McMurray JJ. Neprilysin inhibition to treat heart failure: a tale of science, serendipity, and second chances. Eur J Heart Fail 2015;17(3):242-7. DOI: 10.1002/ejhf.250.
16. Pastormerlo LE, Mammini C, Giannoni A, et al. Glycosylated haemoglobin is associated with neurohormonal activation and poor outcome in chronic heart failure patients with mild left ventricular systolic dysfunction. J Cardiovasc Med (Hagerstown) 2015;16(6):423-30. DOI: 10.2459/JCM.0000000000000159.
17. Nichols GA, Gullion CM, Koro CE, Ephross SA, Brown JB. The incidence of congestive heart failure in type 2 diabetes: an update. Diabetes Care 2004;27(8):1879-84. DOI: 10.2337/diacare.27.8.1879.
18. Greenberg BH, Abraham WT, Albert NM, et al. Influence of diabetes on characteristics and outcomes in patients hospitalized with heart failure: a report from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF). Am Heart J 2007;154(2):277 e1-8. DOI: 10.1016/j.ahj.2007.05.001.
19. Dunlay SM, Givertz MM, Aguilar D, et al. Type 2 Diabetes Mellitus and Heart Failure: A Scientific Statement From the American Heart Association and the Heart Failure Society of America: This statement does not represent an update of the 2017 ACC/AHA/HFSA heart failure guideline update. Circulation 2019;140(7):e294-e324. DOI: 10.1161/CIR.0000000000000691.
20. Luo N, O’Connor CM, Cooper LB, et al. Relationship between changing patient-reported outcomes and subsequent clinical events in patients with chronic heart failure: insights from HF-ACTION. Eur J Heart Fail 2019;21(1):63-70. DOI: 10.1002/ejhf.1299.
21. Velazquez EJ, Morrow DA, DeVore AD, et al. Angiotensin-Neprilysin Inhibition in Acute Decompensated Heart Failure. N Engl J Med 2019;380(6):539-548. DOI: 10.1056/NEJMoa1812851.
22. Nielsen EE, Feinberg JB, Bu FL, et al. Beneficial and harmful effects of sacubitril/valsartan in patients with heart failure: a systematic review of randomised clinical trials with meta-analysis and trial sequential analysis. Open Heart 2020;7(2). DOI: 10.1136/openhrt-2020-001294.
23. Myhre PL, Vaduganathan M, Claggett B, et al. B-Type Natriuretic Peptide During Treatment With Sacubitril/Valsartan: The PARADIGM-HF Trial. J Am Coll Cardiol 2019;73(11):1264-1272. DOI: 10.1016/j.jacc.2019.01.018.
24. Senni M, McMurray JJV, Wachter R, et al. Impact of systolic blood pressure on the safety and tolerability of initiating and up-titrating sacubitril/valsartan in patients with heart failure and reduced ejection fraction: insights from the TITRATION study. Eur J Heart Fail 2018;20(3):491-500. DOI: 10.1002/ejhf.1054.
25. Sokos GG, Raina A. Understanding the early mortality benefit observed in the PARADIGM-HF trial: considerations for the management of heart failure with sacubitril/valsartan. Vasc Health Risk Manag 2020;16:41-51. DOI: 10.2147/VHRM.S197291.