Cardiology
European Heart Rhythm Association (EHRA) 2021 Congress
ETNA-AF Registry: All Atrial Fibrillation Subgroups, Even the Old and Frail, Are Included in NOAC Benefit
Virtual Meeting – Two-year data from the global ETNA-AF registry confirm a remarkably favorable and consistent benefit-to-risk ratio from a non-vitamin K oral anticoagulant (NOAC) across atrial fibrillation (AF) patient groups. In the European subset of the registry, which by itself has followed more than 13,000 patients, the low rates of stroke were accompanied by low rates of major bleeding even among the old and frail. Consistent with the previously completed ENGAGE AF-TIMI 48 trial with the same NOAC, the findings are not a surprise but reinforce the recently revised Canadian guidelines, which now recommend that NOACs be prescribed for most frail elderly patients with AF.
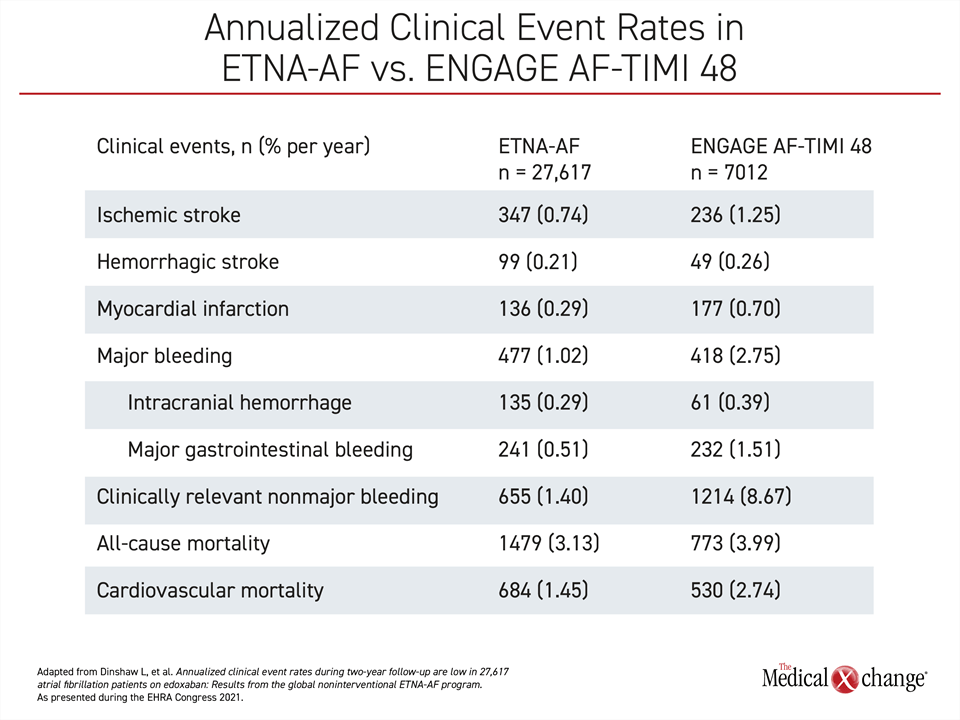
Supporting the previously reported one-year data from a large real-world registry with the NOAC edoxaban, two-year data confirm consistently low rates of stroke and major bleeding, according to Dr. Joris R. de Groot, Head of Clinical Electrophysiology, Amsterdam Medical Center, the Netherlands. Drawn from the 13,417-patient European (ETNA-AF-Europe) registry and full set of 27,617 patients in the ETNA-AF-Global program, the rates reproduce the favorable benefit-to-risk profile reported in the pivotal ENGAGE AF-TIMI 48 (Table 1).
NOAC Benefits Vary Across Trials
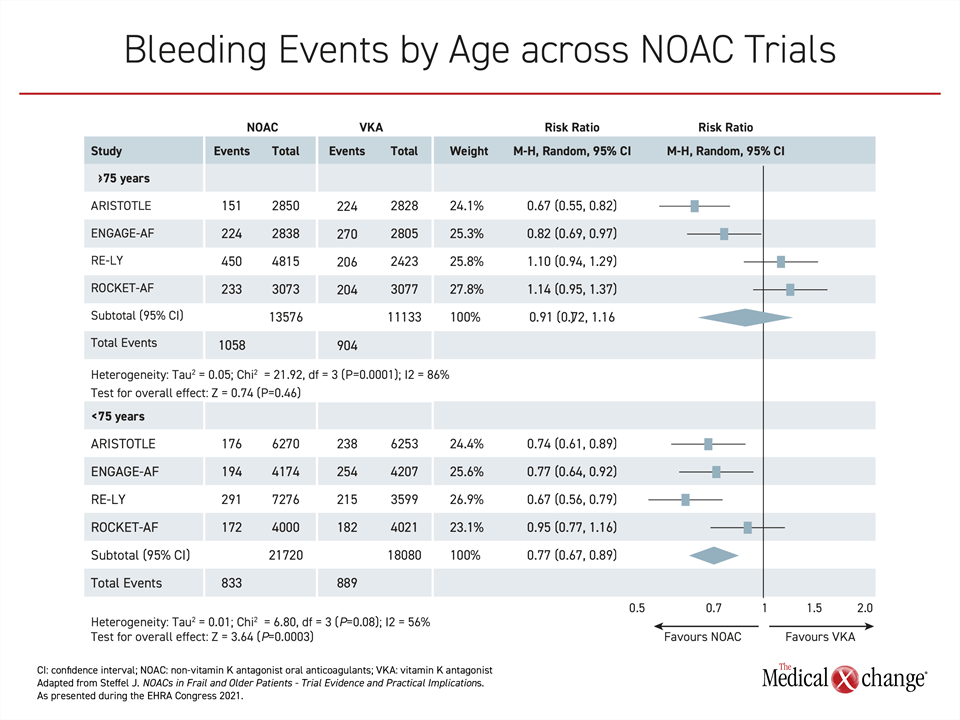
ENGAGE AF-TIMI 48 was one of several NOAC-versus-warfarin non-inferiority trials that led NOACs to be identified as the preferred method of preventing stroke in most AF patients in major guidelines, including those recently revised by the Canadian Cardiovascular Society/Canadian Heart Rhythm Society (CCS/CHRS) (Andrade JG et al. Can J Cardiol 2020;36:1847-1948). In individual trials, not all NOACs achieved a significant safety advantage over warfarin, particularly in the elderly (Figure 1), but there was a favorable general direction for the benefit-to-risk ratio of these therapies, which also have the advantage of being administered without therapeutic monitoring.
In ENGAGE AF-TIMI 48, edoxaban was non-inferior to warfarin (HR 0.79; P<0.001 for non-inferiority) for the primary efficacy endpoint of stroke or systemic embolism. It provided a 13% lower risk (HR 0.87; P=0.005) for a composite endpoint of stroke, systemic embolism, or death from cardiovascular causes. Equally important, edoxaban was associated with a 20% lower risk (HR 0.80; P<0.001) of major bleeding, which was the principal safety endpoint.
The data from ETNA-AF-Global registry now verify that this level of efficacy and safety is sustained over two years in a large real-world dataset.
Recommended vs. Non-Recommended Doses
One explanation for these outcomes is adherence to simple once-daily dosing. At two years, 83% were on the recommended dose, which is 30 mg for those with impaired renal function (<50 mL/min) or low body weight (£60 kg) and 60 mg for all others. In a detailed analysis presented by Dr. de Groot, risks of stroke or bleeding were only modestly elevated for those taking 60 mg when indicated for 30 mg and for those taking 30 mg when indicated for 60 mg, but the sustained adherence of greater than 80% likely accounts for annualized stroke rates of <1.2% and annualized major bleeding rates of <2.0% on any dose calculated at two years.
“The favorable benefit-to-risk ratio was consistent across populations stratified by region or age.”
The favorable benefit-to-risk ratio of edoxaban was consistent across populations stratified by region or age. Globally, ETNA-AF included 11,330 patients from Japan and 2,870 patients from Korea/Taiwan in addition to those from Europe. Efficacy as measured by annualized rates of ischemic stroke, was similar if numerically better for ETNA-AF-Europe relative to ETNA-AF Global (0.51% vs. 0.74%). This was also true of major bleeding (0.97% vs. 1.02%) and intracranial hemorrhage (0.2% vs. 0.29%) even if rates of adverse outcomes were impressively low in all cohorts.
For age, ETNA-AF-Global compared outcomes for patients aged <65, aged ≥65-<75, aged ≥75-<85, and aged ≥85. There were higher annualized rates of bleeding and stroke with greater age, but these could be anticipated from a CHA2DS2-VASc score that rose incrementally for every age group, climbing from 1.1 in those <65 years to 4.4 in those ≥85 years of age, according to Dr. Doralisa Morrone, consultant researcher, University of Pisa, Italy.
There is no control group in ETNA-AF-Global, but rising rates of both stroke and major bleeding with age were attenuated by edoxaban relative to warfarin in ENGAGE AF-TIMI 48. The result was an even greater absolute advantage of edoxaban over warfarin in the elderly than in younger patients.
Frail Elderly May Benefit Most
“In a pre-specified analysis of ENGAGE AF-TIMI 48, we were able to show a greater reduction in bleeding rates and all-cause mortality among frail elderly patients than in non-frail patients,” reported Dr. Jan Steffel, Division of Electrophysiology and Cardiac Services, University Heart Center, Zurich, Switzerland. Referring to an ENGAGE AF-TIMI 48 substudy that employed risk of falling as a surrogate measure of frailty (Steffel J et al. J Am Coll Cardiol. 2016;68:1169-1178), Dr. Steffel emphasized that neither age nor frailty should be a rationale for denying NOAC therapy.
There is a prejudice that strokes are caused by underlying disease while bleeding is caused by the treatment, according to Dr. Leon Dinshaw, Department of Cardiology, Hamburg University, Germany. This characterization is not only wrong but irrelevant. “The ETNA-AF-Global registry shows that edoxaban for stroke prevention in AF patients is both safe and effective,” Dr. Dinshaw said.
Conclusion
The two-year data from the real-world ETNA-AF-Global registry with more than 27,000 patients show the same persistently low rates of stroke and bleeding events previously reported at one year. In the ETNA-AF-Europe registry, with more than 13,000 patients, 83% of patients were taking the recommended dose, but efficacy and safety remained relatively well preserved even among those taking higher or lower than recommended doses. Despite a greater risk of events in the elderly, the ENGAGE AF-TIMI 48 trial demonstrated that these are the patients who derive the greatest relative risk reduction from treatment with edoxaban relative to warfarin.