Cardiology
European Society of Cardiology (ESC) Congress 2021
For Preventing Events after TAVI in Patients with Atrial Fibrillation, NOAC Is a Viable Option
Virtual Meeting – A non-vitamin K oral anticoagulant (NOAC) is an alternative to the vitamin K antagonist warfarin when used selectively for patients with atrial fibrillation (AF) after transcatheter aortic valve implantation (TAVI), according to results of a large randomized trial. Presented at the 2021 ESC Congress, ENVISAGE TAVI-AF is the first trial to demonstrate that a NOAC is non-inferior to warfarin, as per the primary efficacy endpoint of prevention of net adverse clinical events (NACE) over 12 months. NACE was defined as death from any cause, myocardial infarction, ischemic stroke, systemic thromboembolism, valve thrombosis, or major bleeding.
Largest TAVI AF Anticoagulant Trial
This is the largest study yet to evaluate a NOAC for the prevention of complications associated with AF in TAVI patients. It was conducted with edoxaban, an oral, reversible, direct factor Xa inhibitor. In a previously-published multinational trial, this agent was associated with a lower risk of major bleeding than warfarin but similar protection from cardiovascular events in a non-valvular AF. ENVISAGE TAVI-AF is the only trial in which a NOAC has been shown to be non-inferior to warfarin for protection from the complications of AF following TAVI on a basis of statistical significance, reported senior investigator Dr. George D. Dangas, Director of Cardiovascular Innovation, Weiner Cardiovascular Institute, Mount Sinai Medical Center, New York City, NY.
“ENVISAGE TAVI-AF is the only trial in which a NOAC was shown to be non-inferior to warfarin for protection from the complications of AF following TAVI.”
In ENVISAGE TAVI-AF, 1426 patients with AF were randomized to edoxaban or warfarin following TAVI. Antiplatelet therapy was permitted in both arms. The standard dose of edoxaban was 60 mg per day. However, about 45% of patients qualified for the lower dose of 30 mg daily based on a creatinine clearance of 15-50 ml/min, a body weight of ≤60 kg, or use of a concomitant P-glycoprotein inhibitor such as cyclosporine or digoxin. The two groups were well matched for baseline characteristics, including CHA2DS2-VASc score (mean 4.5) and history of coronary artery disease (41%).
Predefined Criteria for Non-Inferiority Met
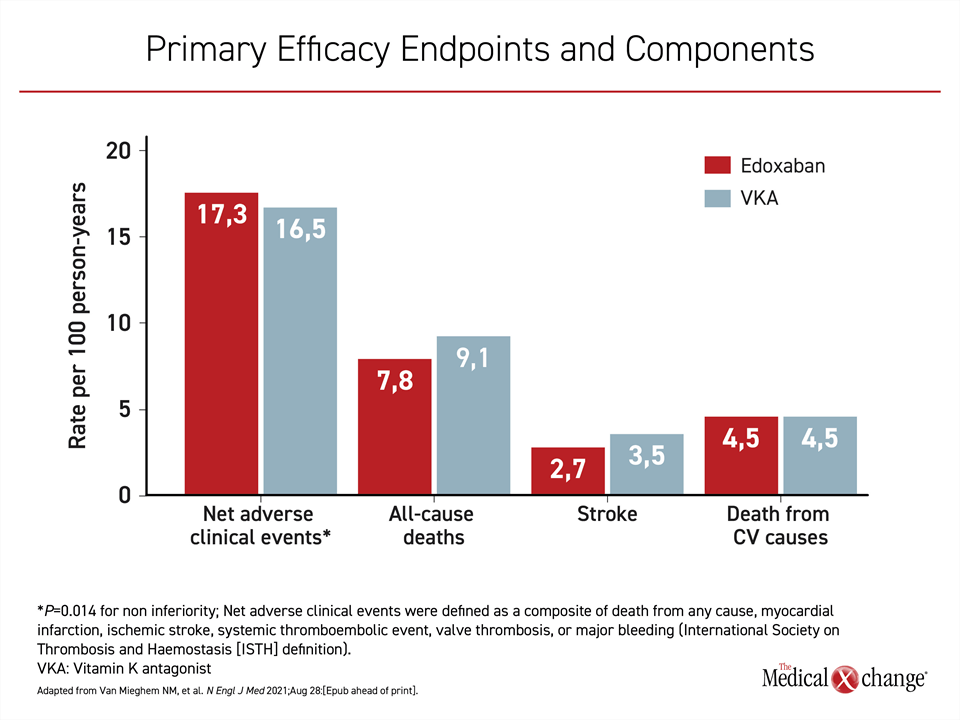
After a mean of 18 months of follow-up, the primary NACE endpoint, expressed per 100 person-years, was reached at rates of 17.3 and 16.5 for edoxaban and warfarin, respectively. These rates did not differ statistically. In addition, edoxaban was found to be non-inferior to warfarin as predefined in the study protocol (significance for non-inferiority: P=0.014). When the components of cardiovascular events were assessed individually, edoxaban had a numerical advantage over warfarin for rates of all-cause death (7.8 vs. 9.1) and stroke (2.7 vs. 3.5) but not for death from a cardiovascular cause (Figure 1).
Safety Outcomes Assessed
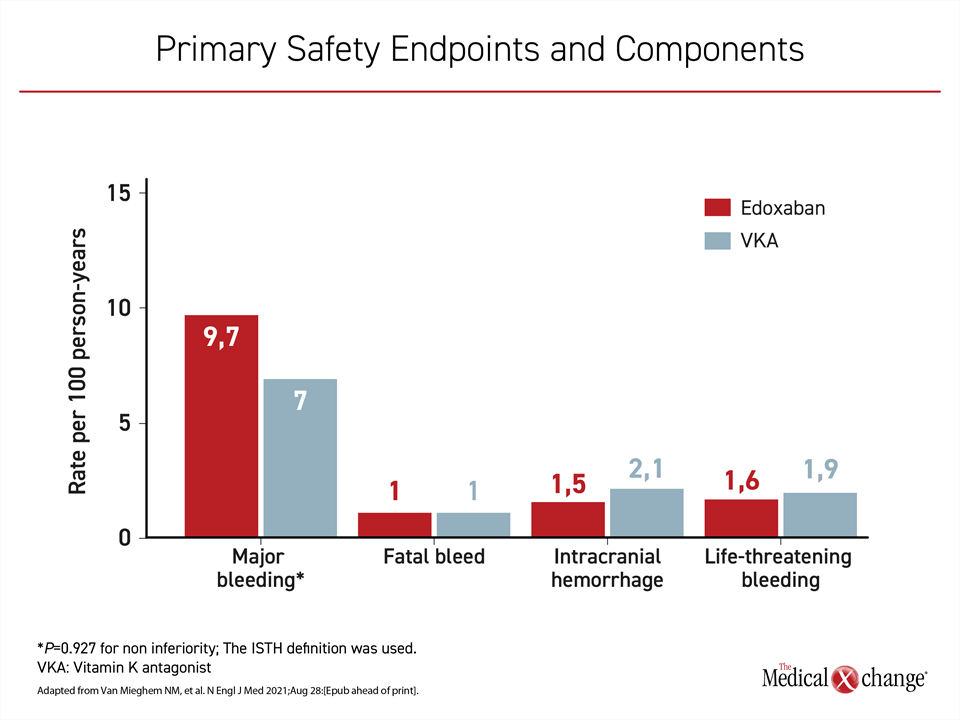
When compared for major bleeding, also expressed as rates per 100 person-years, the higher rate in the edoxaban group (9.7 vs. 7.0) did not meet predefined non-inferiority on a statistical basis (significance for non-inferiority P=0.927). The greater rate of major bleeding in the edoxaban arm was due to a greater rate of gastrointestinal (GI) bleeding, according to Dr. Dangas. The rate of fatal bleeding was identical in the two groups while the rates per 100 person-years of intracranial hemorrhage (1.5 vs. 2.1) and life-threatening bleeding (1.6 vs. 1.9) were numerically lower in the edoxaban group (Figure 2).
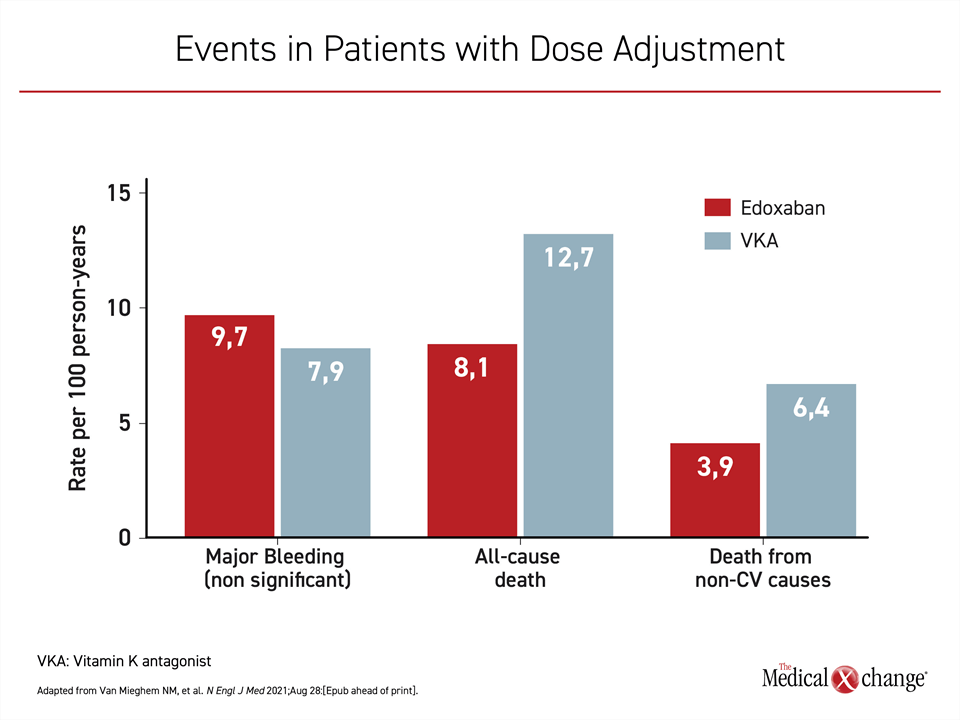
When outcomes were evaluated specifically in those who had an indication for and received the 30 mg dose of edoxaban, the major outcomes were the same except for major bleeding, which was not significantly higher in the edoxaban group (Figure 3).
Unequal Risk Factors for Bleeding
On the primary safety endpoint of major bleeding, the non-inferiority of the NOAC edoxaban was not demonstrated, but the rate of fatal bleeds was the same while the rates of life-threatening bleeds and intracranial hemorrhage were numerically lower on the NOAC.
Several between-group differences might account for the modestly-increased risk of bleeding in the NOAC group, according to Dr. Dangas. One was the higher study drug discontinuation rate in the warfarin group (40% vs. 30%), which means that those on warfarin had less exposure to an anticoagulant. In addition, the mean percent of time in the therapeutic range among patients on warfarin was only 63.5%. A post-hoc analysis also indicated that concomitant use of an antiplatelet increased the bleeding risk more in those on edoxaban than warfarin.
Interpreting the Data
“There are many people already on a NOAC before they undergo TAVI, and I think many of us will keep them on [this treatment] rather than switching to a vitamin K antagonist. We now have data to support this,” said Dr. David Coppodanno, Interventional Cardiologist, University Catania, Italy. Commentary from Dr. Paul Dorian, Professor, Division of Cardiology, University of Toronto, Ontario, noted that although “it will take some time to digest the results of this study,” for a patient already on long-term edoxaban prior to TAVI without evidence of bleeding “I might continue the NOAC,” but he called these data “a challenge to interpret.”
Dr. Nicholas M. Van Mieghem, the first author of ENVISAGE TAVI-AF and an interventionalist, Erasmus Medical Center, Rotterdam, the Netherlands, focused on the practical advantages of edoxaban over warfarin. He concluded that ENVISAGE TAVI-AF supports “edoxaban as an alternative to vitamin K antagonists,” in selected patients, particularly those without major risk factors of GI bleeding.
“This is a scientifically positive trial,” reported Dr. Jean-Philippe Collet, Professor of Cardiology, Hôpital Pitié-Salpêtrière, Paris, France. Principal investigator of ATLANTIS, a trial considered negative when apixaban failed to show an advantage over warfarin for preventing NACE following TAVI, Dr. Collet suggested that differences between NOACs are likely to account for the different results of ENVISAGE TAVI-AF.
Conclusion
ENVISAGE TAVI-AF, the largest multicenter randomized trial to compare a NOAC to a vitamin K antagonist for prevention of thrombotic events following TAVI in patients with atrial fibrillation found edoxaban to be statistically non-inferior to warfarin. The results suggest that this NOAC can be reasonably considered for selective use, particularly among patients who are not at elevated risk of GI bleeding.