Cardiology
Heart Failure: Expert Review and Commentary from Published Literature
Optimizing HFrEF Survival by Early Initiation of All Guideline-Directed Medical Therapies
Anique Ducharme, MD, MSc, FRCP, FACC
Professor of Medicine, Université de Montréal
Director, Heart Failure Clinic
Montréal Heart Institute, Research Center
President, Canadian Heart Failure Society
Montreal, Quebec
Jonathan Howlett, MD, FRCPC, FCCS, FACC, FHFSA (hon)
Clinical Professor of Medicine, University of Calgary
Libin Cardiovascular Institute of Alberta, South Health Campus
Past & Founding President, Canadian Heart Failure Society
Calgary, Alberta
The recently updated Canadian Cardiovascular Society/Canadian Heart Failure Society (CCS/CHFS) guidelines are exclusively on the treatment of patients with heart failure with reduced ejection fraction (HFrEF). In particular, new emphasis has been placed on the importance of starting all four of the first-line guideline-directed medical therapies (GDMT) promptly after diagnosis. As defined in the updated GDMT, the four components are a beta blocker (BB), a mineralocorticoid inhibitor (MRA), an angiotensin receptor neprilysin inhibitor (ARNI), and a sodium-glucose co-transporter 2 (SGLT2) inhibitor. In the previous 2017 guidelines, strong recommendations were made to switch patients from angiotensin receptor blocker (ARB)/angiotensin-converting enzyme (ACE) inhibitor to ARNI, but the uptake of ARNI has been very slow and below expectations despite evidence of important survival benefit. The addition of a fourth recommended class of pharmacological agent make this low uptake of triple therapy worrisome, as failure to provide optimal treatment with GDMT represents an important public health concern. The estimated gains in survival when patients receive all four therapies are measured in years. Conversely, failure to adhere to standards of care means avoidable hospitalizations and deaths.
Definition of GDMT in HFrEF: Guideline Changes
In Canada and elsewhere, HFrEF is a major source of morbidity and mortality consuming a substantial proportion of healthcare costs due to inevitable progression and repeat hospitalizations.1 In patients with HFrEF, defined as a left ventricular ejection fraction (LVEF) of 40% or lower, a series of pharmacologic therapies introduced over the past 30 years have provided incremental, synergistic and substantial protection against disease progression and death. The newly updated CCS/CHFS guidelines have revised the standard.2
There are now four key therapies with an evidence-based survival benefit. In addition, the guidelines include an important reorientation in the algorithm. This update builds upon the previous guidelines published in 2017,3 and now emphasizes initiation and optimization of all the key GDMT agents within three to six months of the HFrEF diagnosis. The recommendation for early start of comprehensive therapy is independent of symptom severity, and it is applicable to most patients.For an exclusive interview with Dr. Jonathan Howlett on the impact to clinical practice, click here
Of the four key GDMT agent classes, the survival benefits of each have been demonstrated on top of the treatment standard at the time that pivotal trials establishing the new or additional standard were conducted2.
In brief, an ACE inhibitor produced a 19% reduction (P=0.019) in all-cause mortality relative to placebo in the landmark SOLVD (Studies of Left Ventricular Dysfunction) trial conducted in the early 1990s.4 Patients in this trial were otherwise on standard of care therapies. The value of adding a BB to an ACE inhibitor was established in several subsequent trials. In CIBIS-II (Cardiac Insufficiency Bisoprolol Study II), for example, there was a 34% (P<0.0001) reduction in all-cause mortality relative to placebo when most patients in both groups were taking an ACE inhibitor.5 Similarly, the survival benefit of MRA therapy was achieved on top of BB and ACE inhibitors. In the EMPHASIS-HF (Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure) trial, for example, this mortality reduction relative to placebo was 24% (P=0.01).6
In the 2017 guidelines, the ARNI sacubitril/valsartan replaced ACE inhibitors as a foundational GDMT in HFrEF patients on the basis of the PARADIGM-HF (Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure) trial. When compared to enalapril in this study, the ARNI provided a 16% reduction (P<0.001) reduction in all-cause mortality relative to an ACE inhibitor when more than 90% in both groups were taking a BB and more than 50% were taking a MRA.7
SGLT2 inhibitors are the most recently added class of GDMT for patients with HFrEF. In one of several trials showing a mortality benefit, dapagliflozin produced a 17% reduction (P<0.01) in all-cause mortality relative to placebo on top of optimal care that included a BB in more than 90% and a MRA in more than 70%.8 Only about 10% of participants in this trial, called DAPA-HF (Dapagliflozin and Prevention of Adverse-Outcomes in Heart Failure), received an ARNI, which was not yet a standard when DAPA-HF was launched, but most not taking an ARNI were taking an ACE inhibitor. In a similar trial called EMPEROR-Reduced (Empagliflozin Outcome Trial in Patients with Chronic Heart Failure and a Reduced Ejection Fraction), about 20% of patients were on an ARNI.9 Subsequent analyses found the benefit from the SGLT2 inhibitor evaluated in this trial, empagliflozin, was similar in those who were or were not on an ARNI.
Each component of GDMT is thought to exert multiple favorable effects on the cardiovascular system that are complementary to each other. While each medication class inhibits HFrEF progression and promotes cardiac remodeling, they do so by affecting different neurohormonal systems.10 For example, BB are thought to act primarily through inhibition of the sympathetic nervous system.11 ACE inhibitors and ARB confer benefits via inhibition of the renin-angiotensin system (RAS).12 In the case of MRA, the target is inhibition of aldosterone, resulting in an antifibrotic effect.13 For ARNI, there is a favorable interaction between the ARB component on RAS inhibition and the sacubitril component on inhibition of neprilysin to lower levels of NT-proBNP and similar compounds, which further promotes vasodilation, enhanced natriuresis, reverse remodeling and reduced cardiac injury.14
The Cumulative Benefits of Achieving Prompt GDMT
The cumulative benefit of GDMT is large. This is not only deduced from the incremental mortality benefits in the pivotal trials, but recent studies quantifying the estimated additive value of GDMT points to impressive risk reductions. For example, when compared to a conventional strategy of ACE inhibitor and BB, the addition of ARNI, MRA, and SGLT2 inhibitors was estimated to provide a 47% reduction (HR 0.53) in all-cause mortality in a cross-trial analysis that accounted for background therapies after a median follow-up of three years.15 The estimated reduction in a composite endpoint of cardiovascular death or hospital admission for heart failure over the same period of time was 62% (HR 0.38).
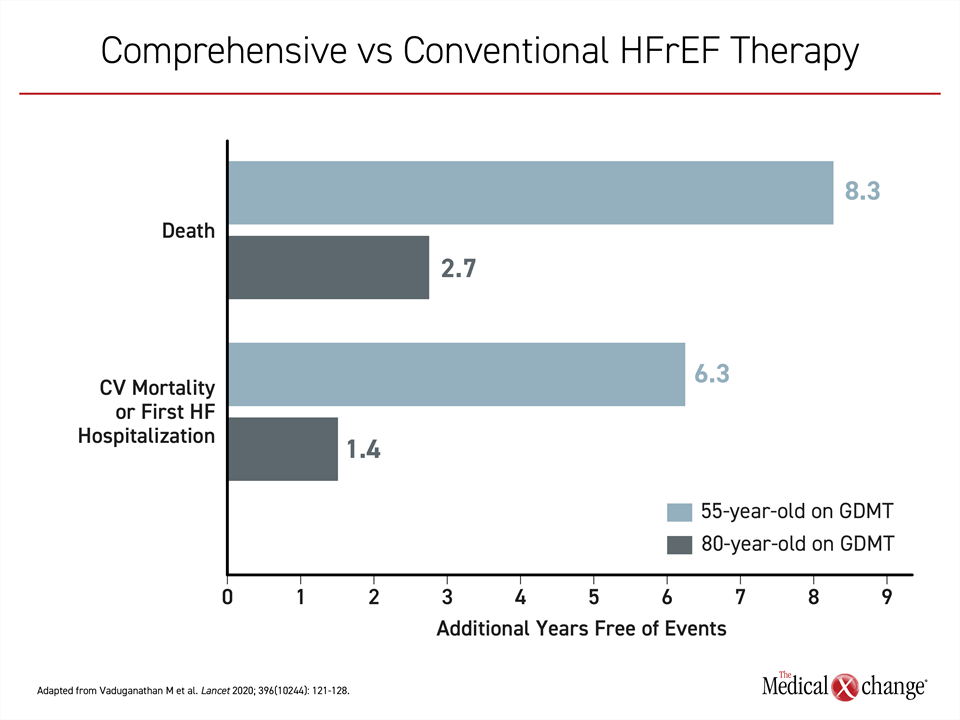
According to modified life-table analyses, a 55-year-old with HFrEF on GDMT relative to conventional therapy could expect 8.3 years of additional life free from cardiovascular death or first hospital admission. For an 80-year old, the estimate was an additional 2.7 years (Figure 1).
A separate analysis calculating cumulative benefits emphasized the incremental importance of combining GDMT.16 Relative to a background of symptom-based therapies such as digoxin, the estimated additive benefit of a conventional triple therapy of ACE inhibitors, BB and MRA was a 56% reduction (HR 0.44) in risk of all-cause mortality. With the addition of ARNI, this relative reduction reached 63% (HR 0.37). A random effects analysis confirmed that each treatment provided an incremental mortality benefit.
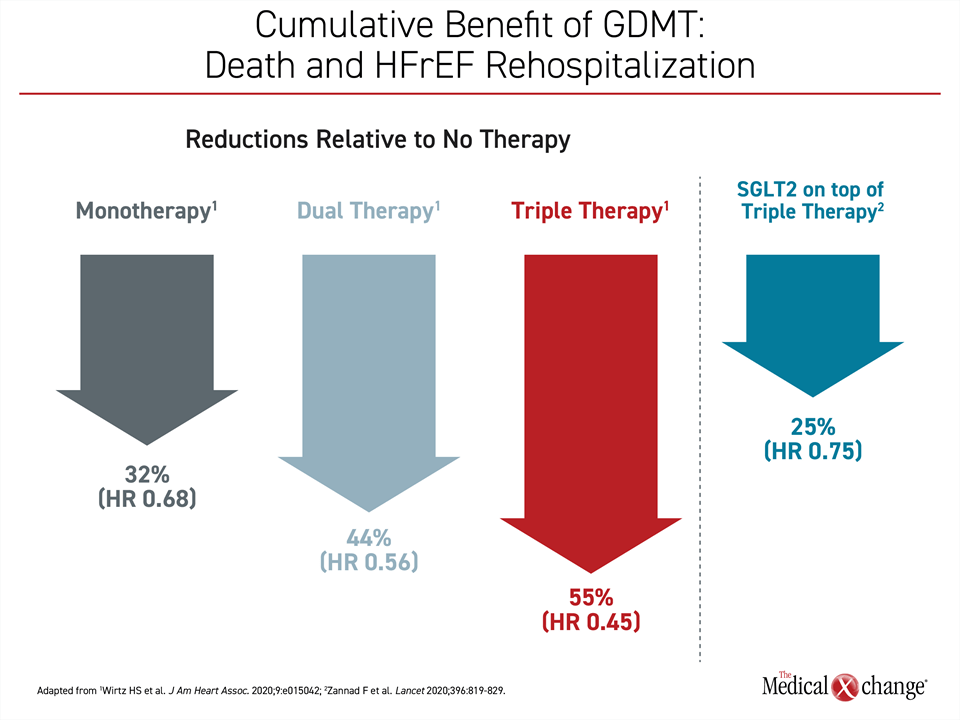
In an outcomes study employing retrospective data from 17,106 HFrEF patients, the same effect was demonstrated.17 The primary outcome evaluated in this study was rehospitalization for heart failure or death one year on treatment after HFrEF hospitalization. Compared to the nearly 4000 patients who received no therapy, a similar sized group receiving just one GDMT had a 32% relative reduction in risk of the primary outcome, rising to a risk reduction of 44% in the more than 7000 patients on two GDMT, and reaching 55% in the 2286 HFrEF patients who received three GDMT therapies. The heart failure trials with SGLT2 inhibitors were generally initiated before or about the time that ARNI was established as one of the pillars of care in HFrEF. As a result, the contribution of SGLT2 inhibitors was evaluated on top of BB, MRA, and either an ACE inhibitor or, in a smaller proportion of patients, an ARNI. The added benefit to triple therapy was large. According to a meta-analysis of the DAPA-HF and EMPEROR-Reduced studies, SGLT2 inhibitors add a further 25% reduction (HR 0.75; P<0.0001) on top of triple therapy for the outcome of cardiovascular death of HFrEF rehospitalization18 (Figure 2).
Suboptimal Care: The Failure to Start ARNI
Within the goal of promptly initiating all GDMT in HFrEF, ARNI therapy deserves particular attention. When introduced in 2017, the CCS/CHFS guidelines recommended a slow titration scheme of the ARNI in stable out-of-hospital patients. Although subsequent studies, such as PIONEER-HF (Comparison of Sacubitril–Valsartan versus Enalapril on Effect on NT-proBNP in Patients Stabilized from an Acute Heart Failure Episode),19 showed that initiation of an ARNI at the index hospitalization is well tolerated and produces more rapid reductions in NT-proBNP, practice survey data continue to indicate that HFrEF candidates are not receiving this therapy in a timely manner.
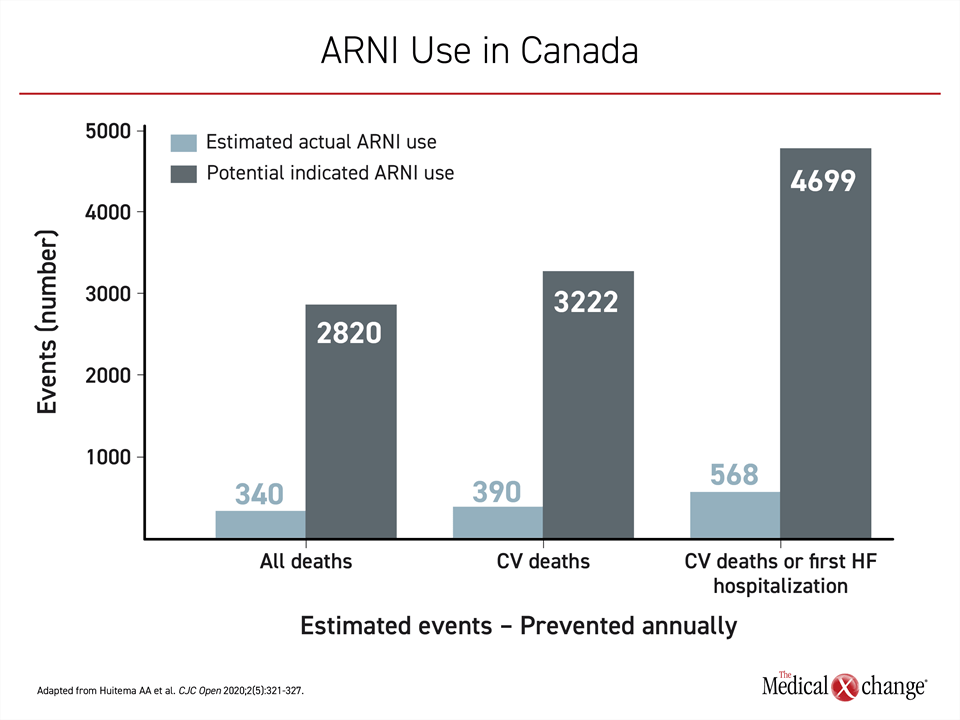
In a 2018 study, for example, only 27,267 (12%) of the 225,562 HFrEF patients in Canada indicated for ARNI were on this therapy.20 While 340 lives were likely saved if the 27,267 HFrEF patients were maintained on ARNI for one year, according to calculations made by the authors on the basis of the pivotal PARADGIM-HF trial, the same calculations predict that 2,820 lives in Canada were lost among the 225,562 HFrEF indicated for this therapy but not treated. Similarly, only an estimated 568 rather than the expected 4,699 HFrEF patients were protected from rehospitalization or death had the ARNI been uniformly prescribed for one year (Figure 3).
To dispel the impression that ARNI is a second-line therapy to be considered only after patients are on a stable dose of BB, MRA, and ACE inhibitor, the new CCS/CHFS guidelines have clearly emphasized that ARNI is a first-line HFrEF treatment that should be initiated in patients admitted to hospital with a new diagnosis of HFrEF. Further, the guidelines clearly indicate the need to switch such patients already treated with an ACE inhibitor or ARB to ARNI prior to hospital discharge.
Very similar language is used in the 2021 American College of Cardiology (ACC) Expert Consensus Decision Pathway.21 Again, the ARNI, like the other three pillars of GDMT, are recommended first line and without delay once a diagnosis of HFrEF has been reached. In these guidelines, initiation of the ARNI along with a BB and MRA is a reasonable first step, although several variables can be considered for individualizing the strategy to ensure that all patients safely reach target doses of all four GDMT components. For most patients, the appropriate target dose is the one proven effective in pivotal trials.
The importance of promptly initiating GDMT and uptitrating to target doses is reflected in the ARNI clinical data. Elevated levels of NT-proBNP are characteristic of HFrEF and indicate both clinical risk and propensity for ongoing left ventricular changes in cardiac geometry and function that represent remodeling. This begins early and progresses over time. In the open-label PROVE-HF (Prospective Study of Biomarkers, Symptom Improvement, and Ventricular Remodeling During Sacubitril/Valsartan Therapy for Heart Failure) study, reductions in NT-proBNP induced by sacubitril/valsartan were associated with reversed cardiac remodeling within six months.22
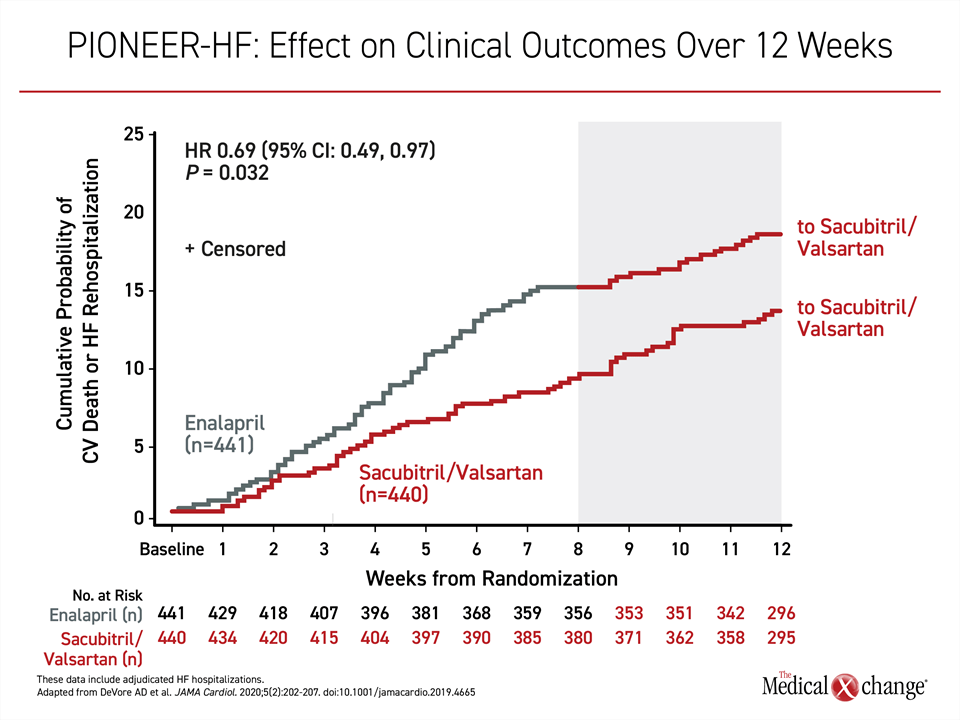
More recently, the PIONEER-HF trial demonstrated that in-hospital initiation of ARNI is safe and results in reduction in NT-proBNP concentrations on ARNI relative to enalapril.19 The separation of the curves for NT-proBNP were seen within one week. Although this short eight-week trial was not designed to show a difference in clinical events, a highly statistically significant difference (P=0.001) in a composite of adverse heart failure events (heart failure rehospitalization, placement of a left ventricular assist device, listing for transplant, or death) favoring the ARNI had already been achieved by eight weeks. The curves remained separated after the eight-week blinded phase (Figure 4).
Implementing the Guidelines
Both the CCS/CHFS and American College of Cardiology (ACC) guidelines outline and address potential obstacles for guideline adherence. One motivation is likely to have been concern about therapeutic inertia. New guidelines can be confusing and intimidating, but both the CCS/CHFS and ACC documents are written with tips and recommendations to simplify and clarify pathways to implementation.
Typically, guidelines are adopted gradually into routine clinical practice over several years. Even specialists appear to exercise caution when treating patients they deem to be at high risk for unexpected adverse events. For example, a Canadian study evaluated treatment in 511 HFrEF patients in a specialist tertiary care heart failure clinic, and showed that GDMT therapies, such as BB (98.6%) and MRA (93.4%), were prescribed at high rates, but approximately one third of patients were not taking optimal doses.23 Suboptimal doses were most commonly identified in older patients or those who had a recent stroke or ischemic attack. In some or most of these cases, lower than recommended doses might have been prudent, but one take home message from this study is that reasons other than clinician inertia can explain guideline-nonadherence, mainly physiological (heart rate, blood pressure, tolerance) and biological (renal function and potassium serum level) limitations to reach target dosage of GDMT.
The need for individualization of therapy, including relatively slow titration or lower than target doses of GDMT, is covered in the guidelines. This includes specific examples as well as guidance for clinicians who are considering referral due to unresolved concerns or uncertainty in patients with clinical characteristics, including comorbidities, that complicate treatment choices. The guidelines also place GDMT in the context of supportive treatments and other appropriate steps to improve outcomes or quality of life.
Perhaps most importantly, these guidelines outline a systematic approach that will ensure that all patients receive, or are at least considered, for GDMT. For the CCS/CHFS guidelines, the stated objective was to help clinicians participate in a paradigm shift to early initiation of a comprehensive GDMT regardless of practice setting.
Conclusion
The first-line treatment of HFrEF now includes four therapies. Rather than added sequentially according to symptoms or disease severity, all should be initiated and titrated to optimal target doses in essentially all patients as quickly as possible after the diagnosis. The goal is to prevent the underlying progression of HFrEF, which includes characteristic cardiac remodeling leading to impaired pump function, symptoms, and events. Each of the four GDMT act on independent pathways of cardiac remodeling, which explains their additive benefits. The ARNI component, once recommended for selected HFrEF patients already on first line GDMT, has been moved forward in the algorithm. In Canada, the expected benefit from tight adherence to GDMT is a reduction in HFrEF-related morbidity and mortality.
References
1. Virani SA, Bains M, Code J, et al. The Need for Heart Failure Advocacy in Canada. Can J Cardiol 2017;33(11):1450-1454. DOI: 10.1016/j.cjca.2017.08.024.
2. McDonald M, Virani S, Chan M, Ducharme A. CCS/CHFS heart failure guidelines update: defining a new pharmacologic standard of care for heart failure with reduced ejection fraction. Can J Cardiol 2021;37:531-546.
3. Ezekowitz JA, O’Meara E, McDonald MA, et al. 2017 Comprehensive Update of the Canadian Cardiovascular Society Guidelines for the Management of Heart Failure. Can J Cardiol 2017;33(11):1342-1433. DOI: 10.1016/j.cjca.2017.08.022.
4. Investigators S, Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991;325(5):293-302. DOI: 10.1056/NEJM199108013250501.
5. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet 1999;353(9146):9-13. (https://www.ncbi.nlm.nih.gov/pubmed/10023943).
6. Zannad F, McMurray JJ, Krum H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011;364(1):11-21. DOI: 10.1056/NEJMoa1009492.
7. McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371(11):993-1004. DOI: 10.1056/NEJMoa1409077.
8. McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med 2019;381(21):1995-2008. DOI: 10.1056/NEJMoa1911303.
9. Packer M, Anker SD, Butler J, et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N Engl J Med 2020;383(15):1413-1424. DOI: 10.1056/NEJMoa2022190.
10. Burchfield JS, Xie M, Hill JA. Pathological ventricular remodeling: mechanisms: part 1 of 2. Circulation 2013;128(4):388-400. DOI: 10.1161/CIRCULATIONAHA.113.001878.
11. Rehsia NS, Dhalla NS. Mechanisms of the beneficial effects of beta-adrenoceptor antagonists in congestive heart failure. Exp Clin Cardiol 2010;15(4):e86-95. (https://www.ncbi.nlm.nih.gov/pubmed/21264074).
12. Werner CM, Bohm M. The therapeutic role of RAS blockade in chronic heart failure. Ther Adv Cardiovasc Dis 2008;2(3):167-77. DOI: 10.1177/1753944708091777.
13. Sica DA. Mineralocorticoid Receptor Antagonists for Treatment of Hypertension and Heart Failure. Methodist Debakey Cardiovasc J 2015;11(4):235-9. DOI: 10.14797/mdcj-11-4-235.
14. Jhund PS, McMurray JJ. The neprilysin pathway in heart failure: a review and guide on the use of sacubitril/valsartan. Heart 2016;102(17):1342-7. DOI: 10.1136/heartjnl-2014-306775.
15. Vaduganathan M, Claggett BL, Jhund PS, et al. Estimating lifetime benefits of comprehensive disease-modifying pharmacological therapies in patients with heart failure with reduced ejection fraction: a comparative analysis of three randomised controlled trials. Lancet 2020;396(10244):121-128. DOI: 10.1016/S0140-6736(20)30748-0.
16. Burnett H, Earley A, Voors AA, et al. Thirty Years of Evidence on the Efficacy of Drug Treatments for Chronic Heart Failure With Reduced Ejection Fraction: A Network Meta-Analysis. Circ Heart Fail 2017;10(1). DOI: 10.1161/CIRCHEARTFAILURE.116.003529.
17. Wirtz HS, Sheer R, Honarpour N, et al. Real-World Analysis of Guideline-Based Therapy After Hospitalization for Heart Failure. J Am Heart Assoc 2020;9(16):e015042. DOI: 10.1161/JAHA.119.015042.
18. Zannad F, Ferreira JP, Pocock SJ, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet 2020;396(10254):819-829. DOI: 10.1016/S0140-6736(20)31824-9.
19. Velazquez EJ, Morrow DA, DeVore AD, et al. Angiotensin-Neprilysin Inhibition in Acute Decompensated Heart Failure. N Engl J Med 2019;380(6):539-548. DOI: 10.1056/NEJMoa1812851.
20. Huitema AA, Daoust A, Anderson K, et al. Optimal Usage of Sacubitril/Valsartan for the Treatment of Heart Failure: The Importance of Optimizing Heart Failure Care in Canada. CJC Open 2020;2(5):321-327. DOI: 10.1016/j.cjco.2020.03.015.
21. Maddox TM, Januzzi JL, Jr., Allen LA, et al. 2021 Update to the 2017 ACC Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment: Answers to 10 Pivotal Issues About Heart Failure With Reduced Ejection Fraction: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol 2021;77(6):772-810. DOI: 10.1016/j.jacc.2020.11.022.
22. Januzzi JL, Jr., Prescott MF, Butler J, et al. Association of Change in N-Terminal Pro-B-Type Natriuretic Peptide Following Initiation of Sacubitril-Valsartan Treatment With Cardiac Structure and Function in Patients With Heart Failure With Reduced Ejection Fraction. JAMA 2019:1-11. DOI: 10.1001/jama.2019.12821.
23. Jarjour M, Henri C, de Denus S, et al. Care Gaps in Adherence to Heart Failure Guidelines: Clinical Inertia or Physiological Limitations? JACC Heart Fail 2020;8(9):725-738. DOI: 10.1016/j.jchf.2020.04.019.