Dermatology
Plaque Psoriasis: Expert Review and Commentary from Published Literature
PURE Registry: Impact of IL-17 Inhibitor Therapy in Plaque Psoriasis
Melinda Gooderham, MSc, MD, FRCPC
Medical Director, SKiN Centre for Dermatology
Principal Investigator, Probity Medical Research
Assistant Professor, Department of Medicine, Queen’s University
Consultant Physician, Peterborough Regional Health Centre
Peterborough, ON
Simon Nigen, MD, FRCPC
Assistant Professor, Skin Allergy Clinic
Verdun Hospital, University of Montreal,
CIUSSS du Centre-Sud-de-l’Île-de-Montréal,
Director, Sima Dermatology Clinic
Montreal, Quebec
When introduced nearly 20 years ago, biologics proved to be a major advance in the treatment of moderate-to-severe plaque psoriasis. The first class of these therapies, tumor necrosis factor inhibitors (TNFi), have now been joined by inhibitors of interleukin-17 (IL-17i), interleukin-23 (IL-23i) and interleukin-12 and -23 (IL-12/-23i). Since the multinational pivotal phase 3 trials were published nearly 8 years ago, the extensive experience with secukinumab, an IL-17i, has been augmented with maturing data from the PURE registry. Recent data from this registry, which has been enrolling psoriasis patients in Canada and Latin America, show that psoriatic lesions on secukinumab treatment resolve more quickly on head and trunk than on limbs, but that disease control is eventually achieved at similar rates across body areas. The registry data also show greater persistence with secukinumab over 42 months of treatment than ixekizumab, an alternative IL-17i. Additionally, objective incremental improvements in disease control, which were still accruing after 2 years and sustained at 42 months, have correlated with better quality of life.
Background
Psoriasis is a chronic autoimmune inflammatory skin disease strongly associated with genetic susceptibility.1 Of the various types of psoriasis, which include guttate, pustular, and erythrodermic forms, plaque psoriasis is the most common, accounting for 90% of cases.2 A population-based study of adults in Ontario identified a cumulative prevalence of 2.54%.3 Males and females were about equally affected. These rates are consistent with those reported in other western countries.1
For an exclusive interview with Dr. Melinda Gooderham on the impact to clinical practice, click here
Although psoriasis is a chronic disease often requiring indefinite periods of treatment, severity at presentation and over time is variable. In one study of 500 patients, 20% had minimal disease activity and about the same proportion had severe activity at the end of 10 years of follow-up.4 The remainder—representing the majority—had moderate-to-severe skin involvement even if symptoms waxed and waned over that 10-year period.
Severity of psoriasis is typically characterized with objective measures, such as body surface area involvement, but these measures do not necessarily capture disease burden. Due to the discomfort and adverse cosmetic impact of skin lesions, particularly those in visible areas, psoriasis can exert a large unfavourable impact on sense of well-being and self-esteem.5 This is not just reflected in diminished quality of life, but active psoriasis is also associated with reduced levels of employment and income.6
Prior to the introduction of biologics, moderate-to-severe psoriasis was not adequately controlled. In a 1000-patient survey conducted in 2005 just before TNFi were widely available, less than 40% of patients responded that their systemic therapy was satisfactory.7
Towards the Path of Disease Control with Biologics
When drugs in the TNFi class were introduced, the rates of disease control achieved were unprecedented. In a pivotal trial with the TNFi etanercept, the PASI 75 at week 12 was 49%,8 a level typically reached in less than 20% of patients treated with methotrexate.9 However, the need for alternative agents in this class and for new classes of biologics was apparent from the beginning. Despite TNFi efficacy, not all patients respond to therapy, and a substantial proportion lose response over time.
Overall, the rate of primary non-response to TNFi is about 20%.10 Although many patients respond to a different TNFi, the efficacy is typically diminished. In one overview, the peak PASI score for a second TNFi, whether the switch is for lack of response or loss of response, is about 25% lower than an initial TNFi (51.4% vs. 67.7%).11 Moreover, disease control on TNFi diminishes over time. In some studies, up to 32% of patients with a PASI 75 response have lost this degree of control within the first year.12
Secukinumab was granted regulatory approval about 5 years ago. Among biologics approved for psoriasis, secukinumab now has the longest follow-up, supplemented by extension data from the phase 3 programs in psoriasis, as well as those for treatment of psoriatic arthritis and ankylosing spondylitis.13 The most recent data was collected in the PURE registry program,14 which now has follow-up extending to 36 months.
Long-Term Outcomes with an IL-17A Inhibitor
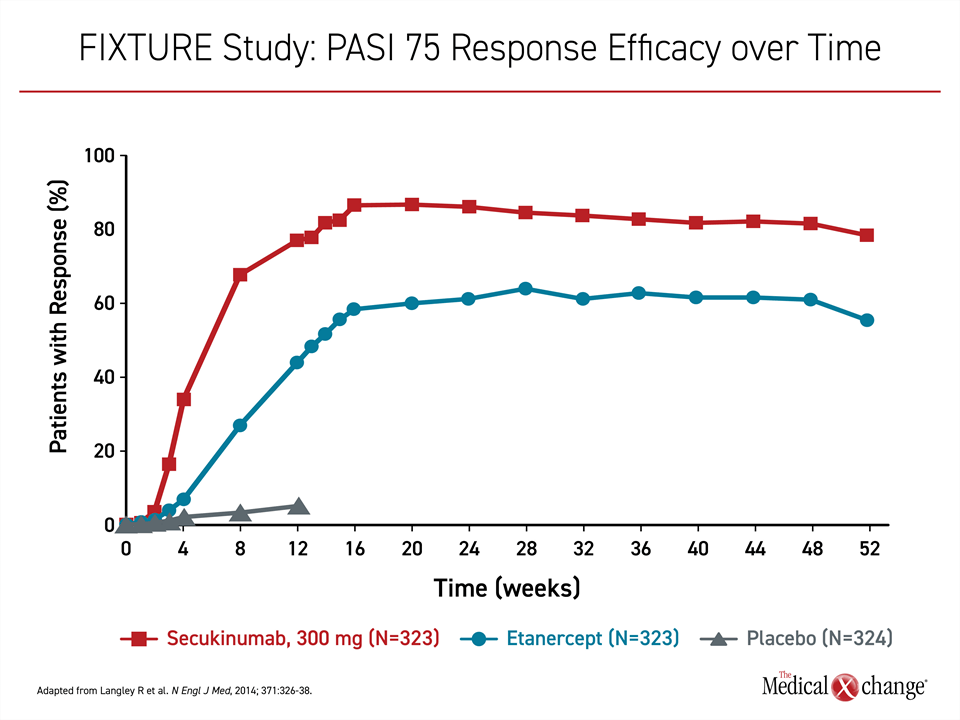
Secukinumab targets IL-17A, a proinflammatory cytokine that has proven targetable in many autoimmune diseases, including psoriatic arthritis and ankylosing spondylitis.15 Following positive phase 2 studies,16,17 the phase 3 trials, ERASURE and FIXTURE, were published together in 2014.18 In ERASURE, 738 patients were randomized to subcutaneous secukinumab or placebo. In FIXTURE, 1306 patients were randomized to secukinumab, placebo, or the TNFi etanercept. With the exception of etanercept, all therapies were administered once weekly for five weeks then every four weeks thereafter. Etanercept was administered twice weekly for 12 weeks then once weekly.
On the now recommended 300-mg dose, secukinumab induced a PASI 75 response in slightly more than 80% of patients in ERASURE and slightly less than 80% in FIXTURE. The response rate on placebo in both studies was <5%. In FIXTURE, the PASI 75 response on etanercept was 44%. The persistent response to secukinumab over 52 weeks was not replicated in the etanercept group (Figure 1).
A similar persistence of benefit was observed in the extension SCULPTURE trial with secukinumab.19 Of 168 patients evaluated at year 1, the PASI 75 response was 88.9%. At year 5, when 126 patients were still evaluable, the PASI 75 response was 88.5%. At both time points, the proportion of patients with a PASI 100 response, signifying the elimination of skin lesions, exceeded 40%.
PURE Registry: Incremental Gains in Disease Control over Time
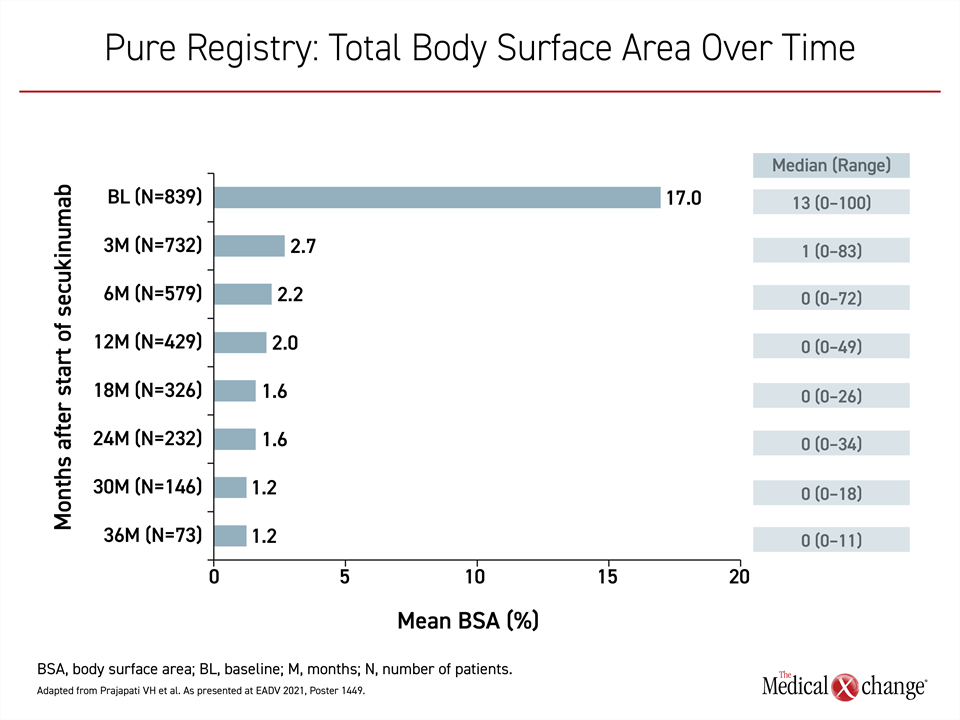
In a series of recent updates from the PURE registry, which has enrolled 2,462 adults with plaque psoriasis from 89 sites in Canada (89%) and Latin America (11%), the data are showing the same degree of sustained efficacy among patients on secukinumab.20 Representing 848 patients (34.5%) of the registry population, the secukinumab patients experienced a major reduction from baseline in every measure of psoriatic involvement, but there were incremental gains over time. The reductions in overall body surface area (BSA) over 36 months were representative of the progressive benefit (Figure 2).
PASI scores for scaling, erythema, and thickening at 3, 6, 12, 18, 24, and 36 months showed the same pattern, with a large immediate reduction from baseline followed by further incremental improvements over time. These improvements were paralleled by patient-completed Psoriasis Symptom Diary (PSD) scores, which covered scaling, cracking, pain, burning, stinging, itching, and skin color. The symptom reductions translated into major improvements in quality of life across multiple domains, including embarrassment, which fell from a baseline score of 5.6 on a scale of 0 to 10, to 1.2 at 6 months, 1.0 at 24 months and 0.8 at 36 months.
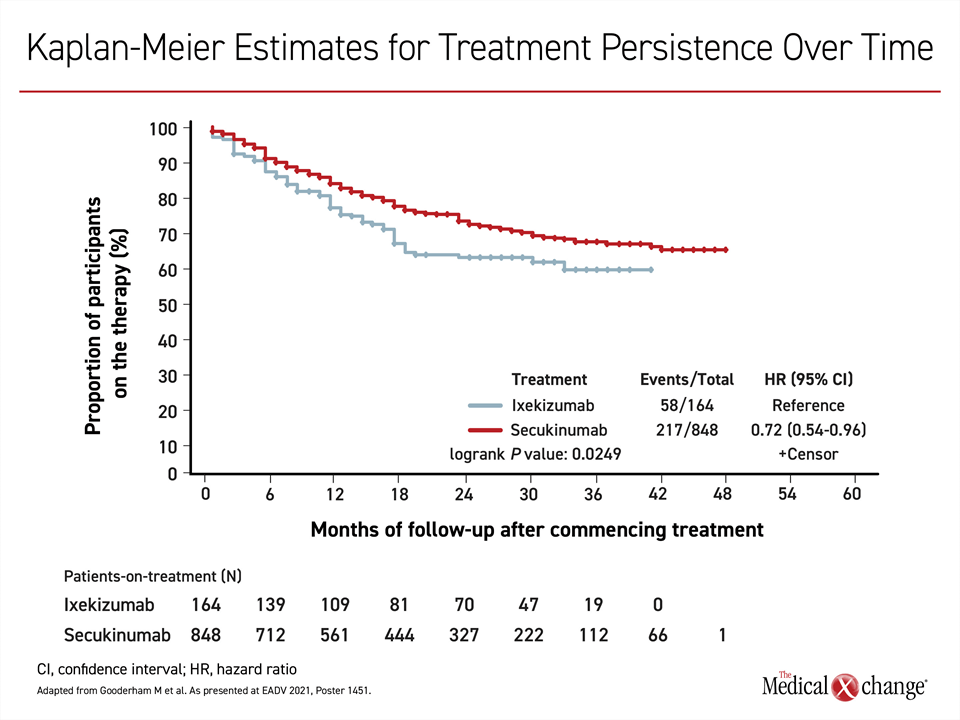
The PURE registry also examined real-world persistence over time in patients on secukinumab relative to ixekizumab.21 Not confined to efficacy, persistence captures tolerability, ease of use, and other variables leading to the ability to remain on therapy. There was 48 months of follow-up among the 848 patients treated with secukinumab and 42 months of follow-up among the 165 patients treated with ixekizumab. Over time, there was a greater loss of persistence in the ixekizumab group even though the dose was uptitrated in a greater proportion of those on ixekizumab vs. secukinumab (36.4% vs. 24.4%) (Figure 3). The most common reason for treatment discontinuation in both groups was loss of treatment efficacy.
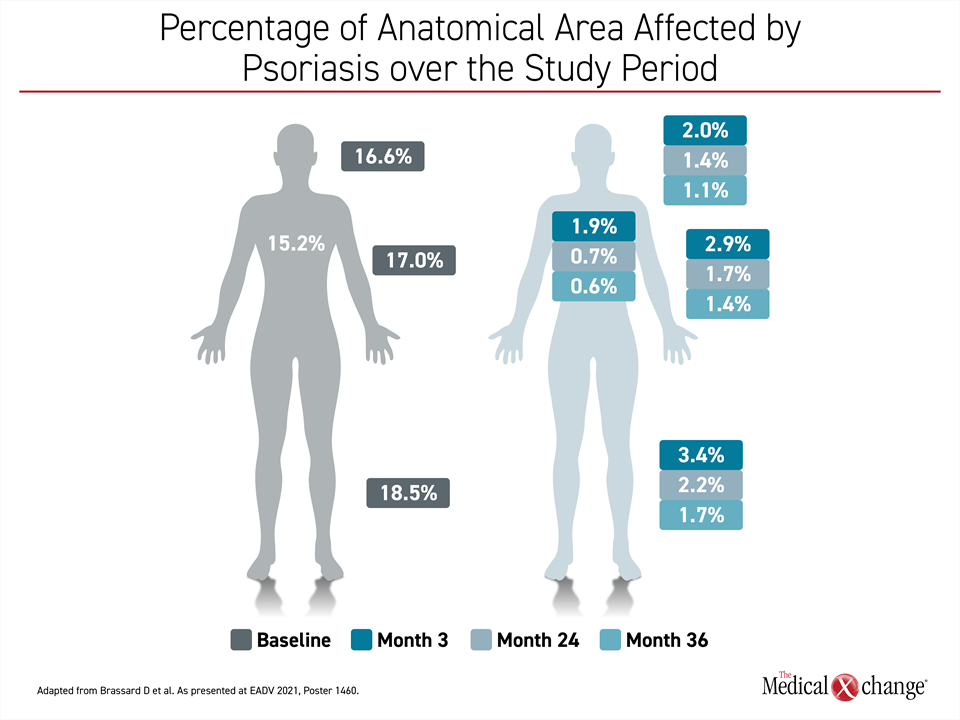
Of the PURE registry updates, the differences in speed of response across anatomical sites in a real-world setting might contain the most important clinical message.22 Relative to resolution of psoriatic lesions on limbs, disease control occurred more rapidly on the trunk, head and neck body regions (Figure 4). For example, PASI regional scores demonstrated improvement by month 3 in the head and neck but were not reached until month 18 in the arms. The same pattern was seen for other scores, such as erythema and scaling, but all scores were low at month 36 even if they were not achieved at the same pace.
For counseling patients, the real-world experience in the PURE registry demonstrates that a steep improvement in the signs and symptoms of psoriasis follows quickly after the initiation of secukinumab, but there is continuous incremental benefit observed up to 3 years. While these data are consistent with the extension studies showing high rates of sustained disease control in patients followed 5 years or more, patients should recognize that improvements do not proceed at the same pace across anatomical sites of disease involvement.
Rationale for Targeting IL-17 in Plaque Psoriasis
The high rates of efficacy associated with secukinumab documented in numerous clinical studies over nearly 10 years of experience were predicted by the mechanism of action. Relative to older biologics, the target of secukinumab, IL-17A, is more specific for psoriasis. The primary effector of Th17 cells,23 IL-17 is released by several types of cells active in psoriatic lesions, including neutrophils and T cells. Once in circulation, IL-17 is a major mediator and orchestrator of the downstream events that characterize psoriatic inflammation.24,25 IL-17 has been characterized as a master cytokine in psoriasis,18 and it has proven to be a targetable driver of other autoimmune diseases including psoriatic arthritis.26 More head-to-head comparisons of biologics in psoriasis are needed, but cumulative data indicate that IL-17i overall, and secukinumab specifically, are effective and well tolerated. Once widely regarded as a second-line biologic, secukinumab is now recommended as a first-line monotherapy for adult patients with moderate-to-severe plaque psoriasis.27
Summary
Double-blind multicenter phase 3 trials play a critical role in the regulatory process. However, extension and registry data are also essential for reaffirming efficacy and safety over time and in evaluating persistence—which is also a factor in sustained disease control. At 42 months, the PURE registry associates secukinumab with incremental gains in disease control over time and after immediate response. It also confirms a high rate of persistence with therapy. Although the speed of improvement varies at specific anatomical sites, high rates of control are achieved with secukinumab across all areas of involvement.
References
1. Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med 2009;361(5):496-509. DOI: 10.1056/NEJMra0804595.
2. Rendon A, Schakel K. Psoriasis Pathogenesis and Treatment. Int J Mol Sci 2019;20(6). DOI: 10.3390/ijms20061475.
3. Eder L, Widdifield J, Rosen CF, et al. Trends in the Prevalence and Incidence of Psoriasis and Psoriatic Arthritis in Ontario, Canada: A Population-Based Study. Arthritis Care Res (Hoboken) 2019;71(8):1084-1091. DOI: 10.1002/acr.23743.
4. Svedbom A, Mallbris L, Larsson P, et al. Long-term Outcomes and Prognosis in New-Onset Psoriasis. JAMA Dermatol 2021. DOI: 10.1001/jamadermatol.2021.0734.
5. Gelfand JM, Feldman SR, Stern RS, Thomas J, Rolstad T, Margolis DJ. Determinants of quality of life in patients with psoriasis: a study from the US population. J Am Acad Dermatol 2004;51(5):704-8. DOI: 10.1016/j.jaad.2004.04.014.
6. Horn EJ, Fox KM, Patel V, Chiou CF, Dann F, Lebwohl M. Association of patient-reported psoriasis severity with income and employment. J Am Acad Dermatol 2007;57(6):963-71. DOI: 10.1016/j.jaad.2007.07.023.
7. Nijsten T, Margolis DJ, Feldman SR, Rolstad T, Stern RS. Traditional systemic treatments have not fully met the needs of psoriasis patients: results from a national survey. J Am Acad Dermatol 2005;52(3 Pt 1):434-44. DOI: 10.1016/j.jaad.2004.10.862.
8. Leonardi CL, Powers JL, Matheson RT, et al. Etanercept as monotherapy in patients with psoriasis. N Engl J Med 2003;349(21):2014-22. DOI: 10.1056/NEJMoa030409.
9. Drach M, Papageorgiou K, Maul JT, et al. Effectiveness of methotrexate in moderate to severe psoriasis patients: real-world registry data from the Swiss Dermatology Network for Targeted Therapies (SDNTT). Arch Dermatol Res 2019;311(10):753-760. DOI: 10.1007/s00403-019-01945-6.
10. Yamauchi PS, Bissonnette R, Teixeira HD, Valdecantos WC. Systematic review of efficacy of anti-tumor necrosis factor (TNF) therapy in patients with psoriasis previously treated with a different anti-TNF agent. J Am Acad Dermatol 2016;75(3):612-618 e6. DOI: 10.1016/j.jaad.2016.02.1221.
11. Ozkur E, Kivanc Altunay I, Oguz Topal I, et al. Switching Biologics in the Treatment of Psoriasis: A Multicenter Experience. Dermatology 2021;237(1):22-30. DOI: 10.1159/000504839.
12. Levin EC, Gupta R, Brown G, Malakouti M, Koo J. Biologic fatigue in psoriasis. J Dermatolog Treat 2014;25(1):78-82. DOI: 10.3109/09546634.2013.826341.
13. Deodhar A, Mease PJ, McInnes IB, et al. Long-term safety of secukinumab in patients with moderate-to-severe plaque psoriasis, psoriatic arthritis, and ankylosing spondylitis: integrated pooled clinical trial and post-marketing surveillance data. Arthritis Res Ther 2019;21(1):111. DOI: 10.1186/s13075-019-1882-2.
14. Papp KA, Gooderham M, Beecker J, et al. Rationale, objectives and design of PURE, a prospective registry of patients with moderate to severe chronic plaque psoriasis in Canada and Latin America. BMC Dermatol 2019;19(1):9. DOI: 10.1186/s12895-019-0087-3.
15. Kuwabara T, Ishikawa F, Kondo M, Kakiuchi T. The Role of IL-17 and Related Cytokines in Inflammatory Autoimmune Diseases. Mediators Inflamm 2017;2017:3908061. DOI: 10.1155/2017/3908061.
16. Papp KA, Langley RG, Sigurgeirsson B, et al. Efficacy and safety of secukinumab in the treatment of moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled phase II dose-ranging study. Br J Dermatol 2013;168(2):412-21. DOI: 10.1111/bjd.12110.
17. Rich P, Sigurgeirsson B, Thaci D, et al. Secukinumab induction and maintenance therapy in moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled, phase II regimen-finding study. Br J Dermatol 2013;168(2):402-11. DOI: 10.1111/bjd.12112.
18. Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med 2014;371(4):326-38. DOI: 10.1056/NEJMoa1314258.
19. Bissonnette R, Luger T, Thaci D, et al. Secukinumab demonstrates high sustained efficacy and a favourable safety profile in patients with moderate-to-severe psoriasis through 5 years of treatment (SCULPTURE Extension Study). J Eur Acad Dermatol Venereol 2018;32(9):1507-1514. DOI: 10.1111/jdv.14878.
20. Prajapati VH, Gooderham M, Beecker J, C. T. Secukinumab treatment results in imporvement of erythema, thickening, and scaling in patients with moderate to severe plaque psoriais: 365-months results fro the PURE registry. European Association of Dermatology and Venerology 2021;Abstract P1499.
21. Gooderham M, Lynde CW, Deforme I, Langley RG. Treatment persistence of secukinumab and ixekizumab in patients with moderate to serere psoriais: results from the PURE Registry. European Association of Dermatology and Venerology 2021;P1451.
22. Brassard D, Papp K, Albrecht L, Maiolino M. Secukinumab treatment shows effectiveness in the treatment of different body regions in patients with moderate to severe psoriasis: 36-month results from the PURE study. European Association of Dermatology and Venerology 2021;P1460.
23. Onishi RM, Gaffen SL. Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease. Immunology 2010;129(3):311-21. DOI: 10.1111/j.1365-2567.2009.03240.x.
24. Harper EG, Guo C, Rizzo H, et al. Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesis. J Invest Dermatol 2009;129(9):2175-83. DOI: 10.1038/jid.2009.65.
25. Martin DA, Towne JE, Kricorian G, et al. The emerging role of IL-17 in the pathogenesis of psoriasis: preclinical and clinical findings. J Invest Dermatol 2013;133(1):17-26. DOI: 10.1038/jid.2012.194.
26. van der Heijde D, Mease PJ, Landewe RBM, et al. Secukinumab provides sustained low rates of radiographic progression in psoriatic arthritis: 52-week results from a phase 3 study, FUTURE 5. Rheumatology (Oxford) 2020;59(6):1325-1334. DOI: 10.1093/rheumatology/kez420.
27. Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol 2019;80(4):1029-1072. DOI: 10.1016/j.jaad.2018.11.057.