Gastroenterology
Ulcerative Colitis: Expert Review and Commentary from Published Literature
Persistence and Adherence to 5-ASA Therapy in Long-Term Control of
Mild-to-moderate Ulcerative Colitis
John Marshall, MSc MD, FRCPC, AGAF, CAGF
Professor of Medicine
Director, Division of Gastroenterology
McMaster University
Hamilton, Ontario
In patients with mild-to-moderate ulcerative colitis (UC), 5-aminosalicylic acid (5-ASA) is an effective first-line option for the goal of clinical and endoscopic remission. To achieve and then maintain this goal, the challenge is strict adherence. In periods of UC quiescence, adherence to daily oral therapies such as 5-ASA commonly declines, allowing disease reactivation. Even if steroids or therapies can restore control, repeated cycles of disease activity can drive progression. There are no studies to indicate that 5-ASA products differ in efficacy. However, the number of pills required per day is not the same. In addition, the mechanisms by which drugs are delivered to the inflamed tissue in the colon differ. To optimize adherence, 5-ASA formulations with simple dosing schemes and effective delivery into the colon should be considered for their practical advantages.
Background
In 2018, the estimated prevalence of inflammatory bowel disease (IBD) in Canada was 0.7%, a rate that represents a substantial rise over the past several decades and that is now among the highest in the world.1 Of the nearly 300,000 cases of IBD, UC represents slightly less than half or about 45%. Both UC and Crohn’s disease are chronic inflammatory diseases of the gastrointestinal tract, but UC is limited to the colon and rectum and is contiguous, whereas Crohn’s can occur throughout the digestive tract with intermittent inflammation between areas of healthy tissue.2 Their symptoms overlap, but rectal bleeding is generally more common with UC.
For an exclusive interview with Dr. John Marshall on the impact to clinical practice, click here
UC severity is defined by such factors as number of bowel movements per day, bleeding, endoscopic appearance, and the presence of elevated inflammatory markers, such as C-reactive protein (CRP).3 In recognizing the substantial differences in the course and treatment of mild-to-moderate relative to moderate-to-severe UC, these strata of severity are typically treated differently. In the case of the American Gastroenterological Association (AGA), guidelines have been issued separately for mild-to-moderate and moderate-to-severe UC.4,5 Unlike moderate-to-severe UC, for which steroids and biologics are used early, 5-ASA remains the mainstay of mild-to-moderate UC management.
Other major guidelines, including those issued by the Canadian Association of Gastroenterology (CAG),6 the American College of Gastroenterology (ACG),3 the British Society of Gastroenterology (BSG),7 and the International Organization for the Study of IBD (IOIBD),8 also identify 5-ASA as the first-line treatment for mild-to-moderate UC.
The shared treatment goal is disease remission, but the definition of remission varies, ranging from absence of clinical symptoms to endoscopic healing. For the CAG, which last issued UC guidelines in 2015, no precise definition was provided for a treatment goal, but the inability to maintain corticosteroid-free remission was identified as a definition of treatment failure. At that time, the CAG guidelines acknowledged emerging evidence of a potential advantage for achieving endoscopic remission, but indicated that more evidence was needed to validate that goal.
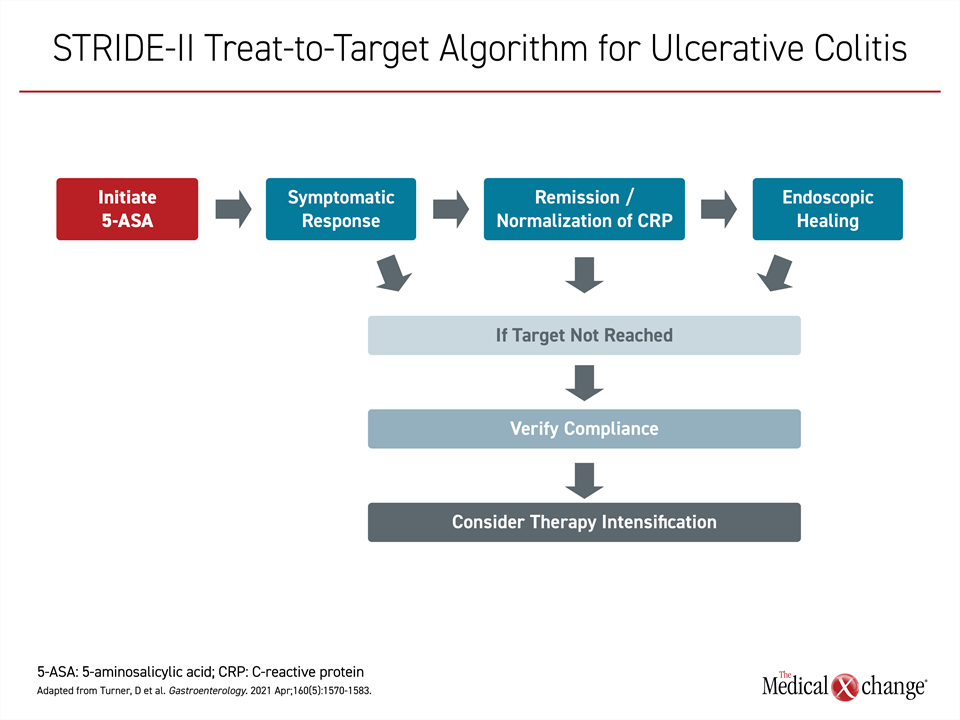
By the time the STRIDE-II recommendations were issued in 2021,8 a treat-to-target approach was advocated with endoscopic remission as the goal based on studies showing better outcomes when this more rigorous definition of remission is achieved. In these guidelines, 5-ASA is the first-line therapy but not the last step if treatment goals are not met. In these guidelines, symptom control is the immediate objective, but endoscopic healing is the long-term target, which if not reached should be addressed with alternative or additional therapies (Figure 1).
Mucosal or endoscopic healing has been variably endorsed in other guidelines, but all recognize the potential for deeper levels of healing to reduce the risk of UC relapse. In order of increasing rigor, these levels of remission are absence of symptoms, mucosal healing or endoscopic remission, and histologic healing. Indeed, the term mucosal healing is now interpreted to encompass resolution of both endoscopic and histologic inflammation. Some guidelines identify other signs of disease control, such as normalization of inflammatory markers, as important intermediate signals of therapeutic response if not an ultimate treatment goal.
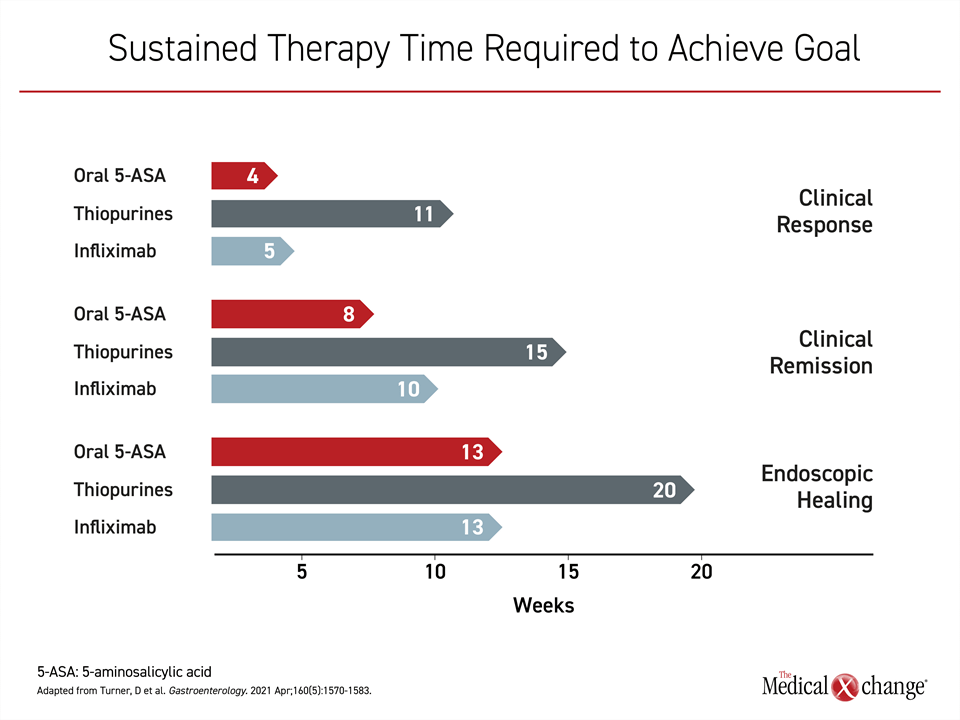
Each of these steps in the healing process in response to 5-ASA or other UC therapies is time dependent. In patients with active disease initiated on 5-ASA, for example, the estimated mean number of weeks to symptom control is 4 weeks, according to an overview in the STRIDE-II guidelines. However, clinical remission, with absence of symptoms of inflammation might require on average an additional 4 weeks. The estimated mean time to full endoscopic healing on 5-ASA therapy is >3 months (Figure 2).
5-ASA Therapies Differentiated
Adherence to treatment is a critical factor and a common challenge for UC and other chronic diseases, according to multiple studies and the World Health Organization.9,10 In the case of UC, extended periods with subclinical disease activity are a contributing factor. When periods of low disease activity eliminate the cue to take therapy at appropriate dose intervals, the defense against poor adherence is simple regimens that are readily incorporated into a daily schedule. Minimizing the pill burden has been shown to be meaningful for chronic diseases in general, and for the prescription of 5-ASA in patients with UC, specifically.11-13
Distinct from sulfasalazine, which is generally less well tolerated than mesalamine,14,15 there are four oral 5-ASA formulations, as well as several rectal formulations including suppositories, enemas and foams available in Canada. There have been no large-scale head-to-head comparisons of these products, but they are considered to be similarly effective and well tolerated for both induction and maintenance on the basis of a systematic analysis of controlled trials.16 However, controlled trials are poorly representative of real-world effectiveness, not least because of the role played by adherence in achieving therapeutic goals. In trials, adherence to 5-ASA commonly exceeds 80%, but some estimates of long-term adherence to 5-ASA in the real world are half that.10
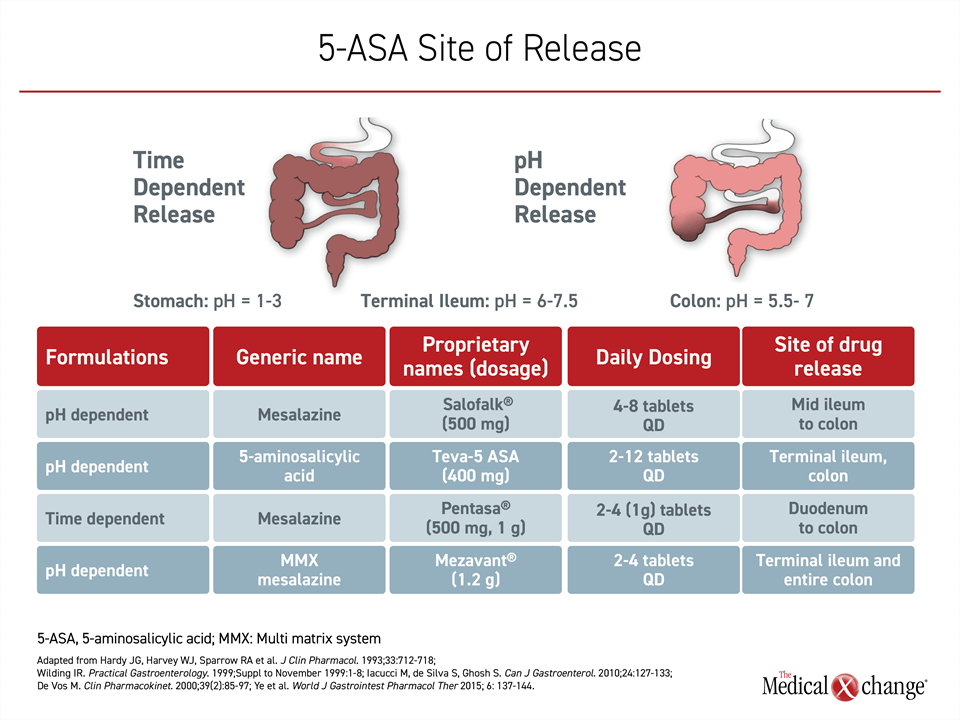
Of the four oral 5-ASA products in Canada, known by the tradenames Pentasa®, Mezavant®, Teva 5-ASA®, and Salofalk®, all but one employ pH-dependent mechanisms to achieve release of the active drug in the colon. The exception, Pentasa, is time dependent. While pH-dependent delivery means that most drug is released when the pills or capsules reach the terminal ileum,17 time-dependent delivery avoid potential intra- and inter-patient variability in luminal pH. The goal of time-dependent delivery is to achieve continuous release as the therapy moves through the gastrointestinal tract (Figure 3). An increase in dosing frequency and a greater number of pills at each dose are negatively associated with long-term adherence.18
More consistent delivery of active drug to the site of the disease is likely to contribute to greater efficacy, but there are no direct studies linking relative levels of 5-ASA in the colon with improved outcomes. However, the most effective therapies cannot yield benefit if drug exposure is not maintained with routine dosing. The relative importance of strict adherence increases with treatment goals, such as sustained mucosal healing, that cannot be reached without sustained periods of disease suppression.
Achieving and Maintaining Remission in Mild-to-Moderate UC
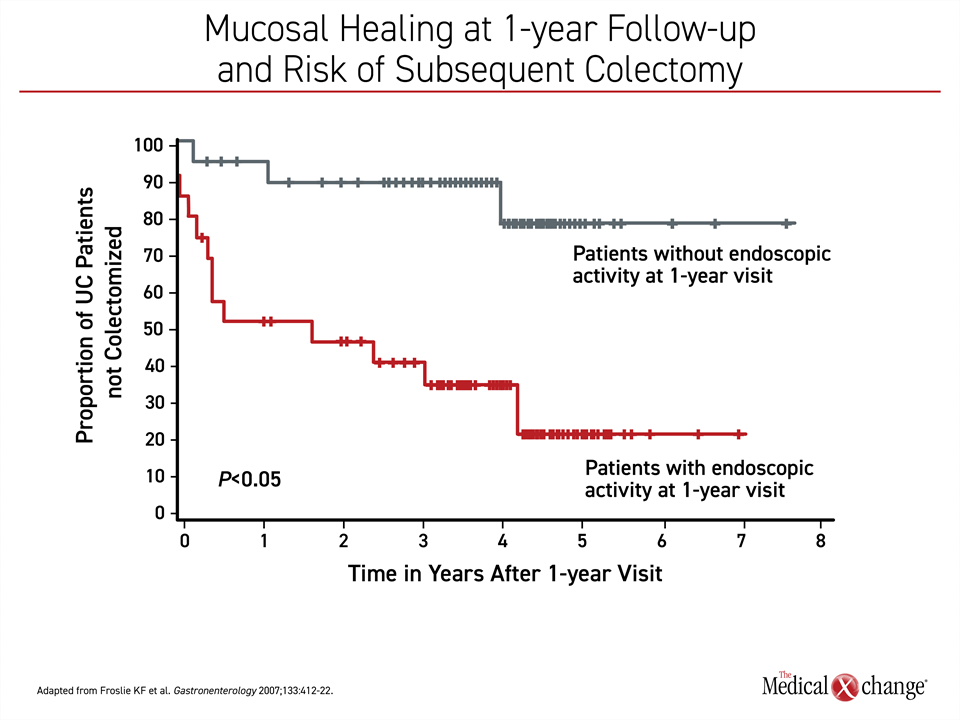
As reflected in the STRIDE-II guidelines, the growing emphasis on treating to a target of disease quiescence, defined as mucosal or endoscopic healing, reflects the concept that deep control of inflammation will lead to longer term sustained remission. In published studies, mucosal healing has predicted fewer surgeries, fewer hospitalizations, and longer periods of remission.19 It has also been shown to translate into an improved quality of life.20 In a study that followed patients with mucosal healing at the end of 1 year of treatment, the proportion of patients who underwent colectomy over 7 years of subsequent follow-up was reduced dramatically (~20% vs. >70%)21 (Figure 4).
The efficacy of 5-ASA in achieving treatment targets in mild-to-moderate UC is well recognized. Based on published trials, the CAG, AGA, ACG, and BGS guidelines have all issued a strong recommendation for 5-ASA as a first-line therapy. Although the addition of corticosteroids can be considered in those not achieving adequate relief on first-line 5-ASA treatment, switching or intensifying therapy should be undertaken only after ensuring that patients have been compliant with their assigned treatment. Guidelines from the CAG identify once-daily dosing formulations to be as effective as formulations requiring divided doses.
Mild-to-moderate UC has a heterogeneous presentation and course. For mild left-sided colitis, oral therapy alone can often be sufficient if patients adhere adequately to the treatment protocol. For more extensive involvement, including proctitis, the addition of rectal 5-ASA or short course of corticosteroids might be required for the treat-to-target goals outlined by current guidelines. In these cases, too, simple regimens of oral 5-ASA remain a backbone of a strategy to gain and maintain remission.
Summary
For mild-to-moderate UC, the major guidelines agree on 5-ASA therapy as the standard for induction and maintenance. The treat-to-target orientation adopted by many guidelines is dependent on the premise that symptom control is important but insufficient. Deeper levels of disease control represented by suppression of subclinical inflammatory activity have been linked to improved outcomes but are dependent on sustained long-term adherence. Patients should be engaged in their own care by understanding treatment goals and the critical aspect of remaining on therapy even in the absence of symptoms, a step facilitated by simple regimens incorporated into a daily schedule.
References
1. Kaplan GG, Bernstein CN, Coward S, et al. The Impact of Inflammatory Bowel Disease in Canada 2018: Epidemiology. J Can Assoc Gastroenterol 2019;2(Suppl 1):S6-S16. DOI: 10.1093/jcag/gwy054.
2. Chang JT. Pathophysiology of Inflammatory Bowel Diseases. N Engl J Med 2020;383(27):2652-2664. DOI: 10.1056/NEJMra2002697.
3. Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. ACG Clinical Guideline: Ulcerative Colitis in Adults. Am J Gastroenterol 2019;114(3):384-413. DOI: 10.14309/ajg.0000000000000152.
4. Ko CW, Singh S, Feuerstein JD, et al. AGA Clinical Practice Guidelines on the Management of Mild-to-Moderate Ulcerative Colitis. Gastroenterology 2019;156(3):748-764. DOI: 10.1053/j.gastro.2018.12.009.
5. Feuerstein JD, Isaacs KL, Schneider Y, et al. AGA Clinical Practice Guidelines on the Management of Moderate to Severe Ulcerative Colitis. Gastroenterology 2020;158(5):1450-1461. DOI: 10.1053/j.gastro.2020.01.006.
6. Bressler B, Marshall JK, Bernstein CN, et al. Clinical practice guidelines for the medical management of nonhospitalized ulcerative colitis: the Toronto consensus. Gastroenterology 2015;148(5):1035-1058 e3. DOI: 10.1053/j.gastro.2015.03.001.
7. Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019;68(Suppl 3):s1-s106. DOI: 10.1136/gutjnl-2019-318484.
8. Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology 2021;160(5):1570-1583. DOI: 10.1053/j.gastro.2020.12.031.
9. Action SEAtL-TTEF. Adherence to Long-Term Therapies: Evidence For Action. World Health Organization 2003; https://who.int/chp/knowledge/publications/adherence_full_report.pdf:Accessed December 7, 2021.
10. Testa A, Castiglione F, Nardone OM, Colombo GL. Adherence in ulcerative colitis: an overview. Patient Prefer Adherence 2017;11:297-303. DOI: 10.2147/PPA.S127039.
11. Farrell B, French Merkley V, Ingar N. Reducing pill burden and helping with medication awareness to improve adherence. Can Pharm J 2013;146:262-269.
12. Kane SV. Strategies to improve adherence and outcomes in patients with ulcerative colitis. Drugs 2008;68(18):2601-9. DOI: 10.2165/0003495-200868180-00006.
13. Lee J, Jee SR, Kim HW, et al. Factors associated with low adherence to oral 5-aminosalicylic acid in patients with ulcerative colitis. PLoS One 2019;14(3):e0214129. DOI: 10.1371/journal.pone.0214129.
14. Ransford RA, Langman MJ. Sulphasalazine and mesalazine: serious adverse reactions re-evaluated on the basis of suspected adverse reaction reports to the Committee on Safety of Medicines. Gut 2002;51(4):536-9. DOI: 10.1136/gut.51.4.536.
15. Sehgal P, Colombel JF, Aboubakr A, Narula N. Systematic review: safety of mesalazine in ulcerative colitis. Aliment Pharmacol Ther 2018;47(12):1597-1609. DOI: 10.1111/apt.14688.
16. Murray A, Nguyen TM, Parker CE, Feagan BG, MacDonald JK. Oral 5-aminosalicylic acid for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev 2020;8:CD000544. DOI: 10.1002/14651858.CD000544.pub5.
17. Iacucci M, de Silva S, Ghosh S. Mesalazine in inflammatory bowel disease: a trendy topic once again? Can J Gastroenterol 2010;24(2):127-33. DOI: 10.1155/2010/586092.
18. Ingersoll KS, Cohen J. The impact of medication regimen factors on adherence to chronic treatment: a review of literature. J Behav Med 2008;31(3):213-24. DOI: 10.1007/s10865-007-9147-y.
19. Reinink AR, Lee TC, Higgins PD. Endoscopic Mucosal Healing Predicts Favorable Clinical Outcomes in Inflammatory Bowel Disease: A Meta-analysis. Inflamm Bowel Dis 2016;22(8):1859-69. DOI: 10.1097/MIB.0000000000000816.
20. Lichtenstein GR, Ramsey D, Rubin DT. Randomised clinical trial: delayed-release oral mesalazine 4.8 g/day vs. 2.4 g/day in endoscopic mucosal healing–ASCEND I and II combined analysis. Aliment Pharmacol Ther 2011;33(6):672-8. DOI: 10.1111/j.1365-2036.2010.04575.x.
21. Froslie KF, Jahnsen J, Moum BA, Vatn MH, Group I. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology 2007;133(2):412-22. DOI: 10.1053/j.gastro.2007.05.051.