Oncology
Prostate Cancer: Expert Review and Commentary from Published Literature
Optimizing the Benefit-to-Risk Ratio of Androgen Deprivation Therapy in Prostate Cancer
Dr. Laurence Klotz, FRCSC, CM
Professor of Surgery, University of Toronto
Sunnybrook Chair of Prostate Cancer Research
Chairman, SIU UCare Research Office
Chairman, Canadian Urology Research Consortium
Sunnybrook Health Sciences Centre
Toronto, Ontario
Dr. Carole Lambert, FRCPC, MA(ed)
Radiation Oncologist, Centre hospitalier de l’Université de Montréal (CHUM)
Full Professor, Department of Radiology, Radio-Oncology and Nuclear Medicine
Associate Vice Dean, Postgraduate Medical Education, Educational Affairs
Université de Montréal,
Montreal, Quebec
Special section on The Cardiologist’s Perspective
Dr. Darryl P. Leong, MBBS (Hons), MPH, M.Biostat PhD, FRACP, FESC
Scientist, Population Health Research Institute
Associate Professor of Medicine, Division of Cardiology
Associate Member, Dept. of Health Research Methods, Evidence, and Impact
Director, Cardio-Oncology Program, McMaster University and Hamilton Health Sciences
Director, Internal Medicine Resident Research
Hamilton, Ontario
The evaluation and treatment of cardiovascular (CV) risk factors in men with prostate cancer has been addressed by national guidelines in many countries. Prostate cancer, like CV disease, is age-related. For individuals with prostate cancer who also have CV disease, these two conditions are coexisting threats to survival. This is a particularly important consideration when prostate cancer is treated with androgen deprivation therapy (ADT). ADT is a standard of care for prostate cancer but exacerbates CV risks. Treatment decisions involve balancing risks and benefits. GnRH antagonists have been associated with lower risk of CV events than GnRH agonists in several studies. Guidelines in Canada have suggested that GnRH antagonists may be preferable in men with prior CV events. Given the difference in activity of these two approaches to hormone suppression, the selection of ADT with a lesser impact on CV disease is reasonable in men with a prior history of CV disease, due to the higher likelihood of further CV events.
Background
CV disease and prostate cancer are closely associated. The risk of both begins to climb at about 50 years of age, and they share multiple risk factors. These include the metabolic syndrome and its related abnormalities, such as reduced insulin sensitivity, increased levels of serum triglycerides, and central adiposity.1,2 The presence of metabolic syndrome is associated with higher odds of being diagnosed with prostate cancer. Increased number and severity of components of the metabolic syndrome correlate with a higher grade of prostate cancer and greater risk of progression.3 Two publications from the Canadian Urological Association (CUA) within the last 2 years have emphasized the importance of evaluating CV risk in patients with prostate cancer and addressing both conditions.1,4
For an exclusive interview with Dr. Laurence Klotz on the impact to clinical practice, click here
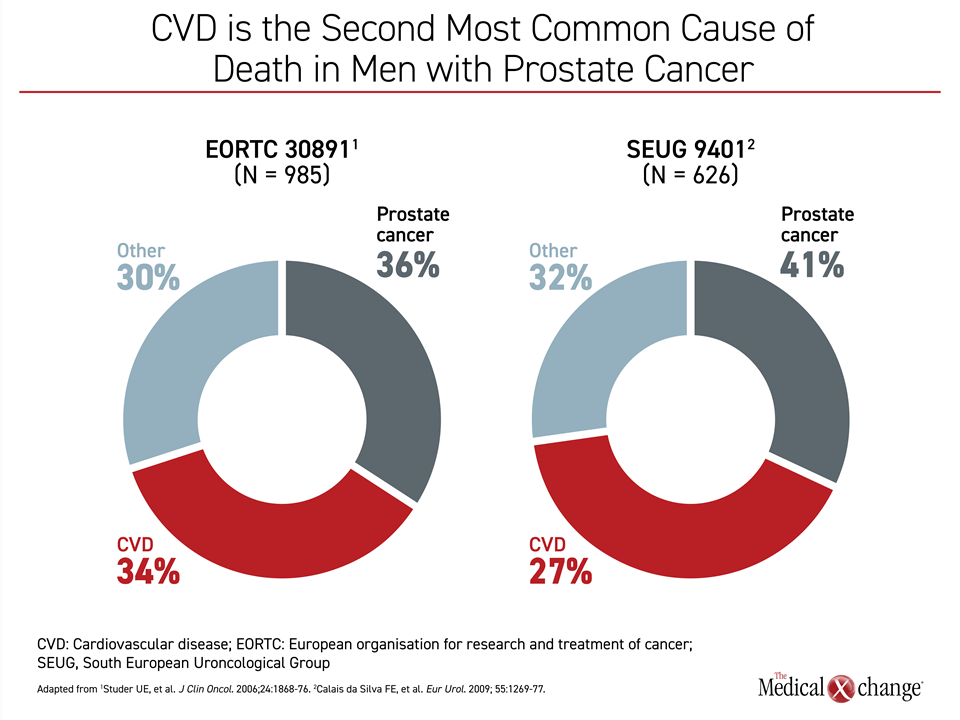
Prostate cancer is the most frequent cancer diagnosed in men and one of the most common causes of cancer death.5 Many of these cancers are indolent.6 Even as prostate cancer advances, non-cancer-related causes of death, particularly those due to CV events, are common. In one US-based study of 29,000 men with local or regional prostate cancer followed over time, CV disease edged out prostate cancer as the cause of death (23% vs. 17%).7 Other studies of localized prostate cancer have also demonstrated that the percentage of death from a CV cause exceeds that of death from the malignancy (Figure 1).
In metastatic prostate cancer, the majority of deaths are cancer-related, but CV events remain the most common cause of non-cancer death and negatively affect survival.8
In one of two CUA publications on the topic of prostate cancer and CV risk, a multidisciplinary team of Canadian physicians described the rationale for routine screening of CV risk factors and treatment in patients with prostate cancer.1 In this review, it was emphasized that CV risk assessment and modification is appropriate in individuals being considered for ADT. The other CUA publication provided guidelines on managing adverse events in ADT, many of which, such as metabolic changes and alterations in body composition, directly or indirectly increase risk of CV complications.4
ADT in Prostate Cancer
In unresectable prostate cancer, ADT has numerous indications, including those involving localized, metastatic, and recurrent disease.9,10 In patients with localized disease, ADT can improve survival when used adjunctively with radiation.11 In those with high-risk disease, courses of 2 to 3 years have been associated with a reduced risk of recurrence.9 In advanced prostate cancer, including recurrent localized prostate cancer or non-metastatic localized cancer not suitable for curative treatments, ADT can be effective for symptom control and slowing progression. However, ADT is most commonly used in advanced and metastatic prostate cancer.9
The efficacy of ADT is derived from the role played by activation of androgen signaling in malignant prostate cell growth.12 Among numerous hormones that participate in prostate gland function, testosterone and dihydrotestosterone stimulate growth of malignant cells in the prostate. In prostate cancer, benefit is derived from suppressing the activity of these hormones. This can be achieved by bilateral orchiectomy, exogenous administration of gonadotropin-releasing hormone (GnRH) agonists or antagonists, or treatments targeted at signaling pathways through blockade of receptors.10
Both antagonists and agonists of GnRH, also known as luteinizing hormone-releasing hormone (LHRH), inhibit release of testosterone. The mechanisms of action of these 2 classes of drugs differ. Antagonists, which provide a direct blockade of GnRH receptors, produce a more rapid suppression of testosterone than agonists.13 In comparison, agonists trigger an initial testosterone surge (and microsurge on repeat dosing) before producing receptor desensitization and downregulation of testosterone.14
ADT can be effective for preventing relapse but is not usually curative. Rather it is used to prevent recurrence or inhibit progression. Both GnRH agonists or antagonists are effective therapies when ADT is indicated, according to several guidelines, including those issued by the National Comprehensive Cancer Network (NCCN).15 Both GnRH antagonists and agonists are considered appropriate for clinically localized disease, regional disease, recurrent disease after radiation or prostatectomy, and in metastatic disease.
In Canada, approved GnRH agonists include leuprolide, goserelin, and triptorelin. The only approved GnRH antagonist is degarelix. Consistent with several comparative trials of GnRH antagonists and agonists, degarelix was shown to be as effective as the GnRH agonist leuprolide for suppressing testosterone over 1 year.16 In that trial, the proportion of patients achieving castration-level suppression of testosterone by day 14 was substantially higher on degarelix (100% vs. 18.2%). Over the course of the trial, degarelix, unlike leuprolide, was not associated with testosterone microsurges with repeat dosing.
ADT and CV Risk Management in Prostate Cancer
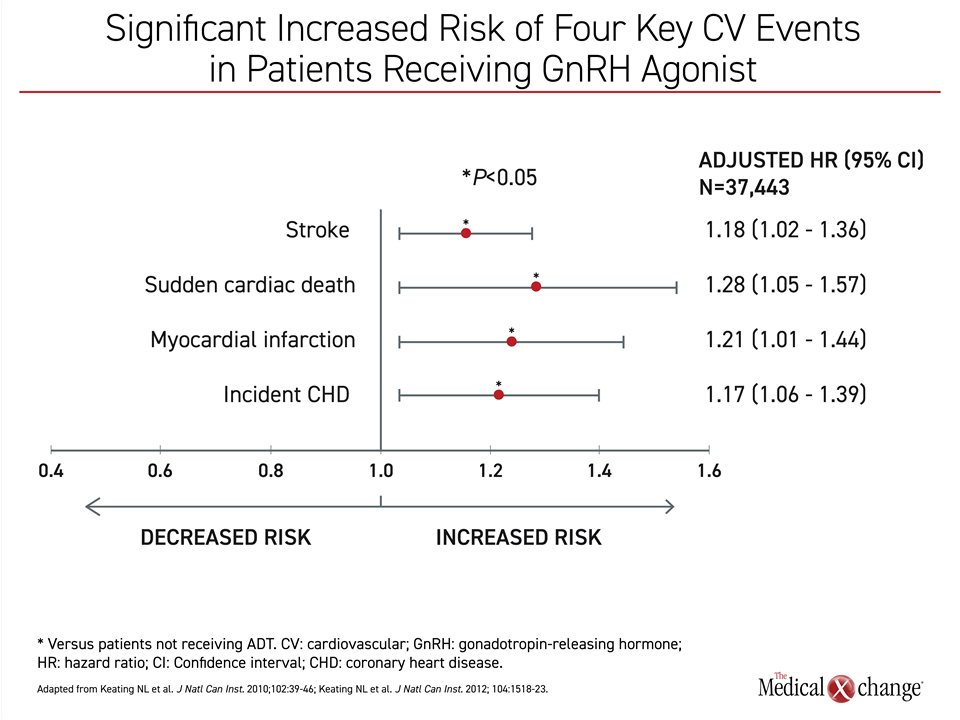
The relationship between ADT and CV risk is well established. In an observational study that included more than 37,000 prostate cancer patients, ADT with either orchiectomy or GnRH agonists produced an increased risk of CV disease and events.17 For orchiectomy, the significant risks included new onset coronary heart disease (CHD) and myocardial infarction (MI). For GnRH agonists, the risks included stroke, sudden cardiac death, MI, and new onset CHD (Figure 2). On the basis of this and other studies, Health Canada adjusted the labelling of GnRH agonists in 2011 to include increased CV risks. In the guidelines from the CUA on use of ADT, specific strategies are outlined for the modification of CV risk in patients placed on ADT, including baseline CV risk assessment and management of CV risk factors. These guidelines call for primary care physicians to be notified when ADT is started.
There is now substantial evidence suggesting that GnRH antagonists pose a lower CV risk than GnRH agonists. The most compelling evidence is in patients with established CV disease. In a 2014 pooled analysis of 6 randomized trials in men with prostate cancer and a history of CV disease, those treated with a GnRH antagonist were compared to those receiving a GnRH agonist for a composite endpoint that included MI, thrombotic events, and CV death. Despite a similar baseline risk profile, the hazard ratio (HR) for these events in men with pre-existing CV disease was more than 50% lower risk for antagonists relative to agonists (HR 0.44; P=0.002).18
Two more recently conducted randomized trials comparing a GnRH antagonist to a GnRH agonist also showed greater CV safety for the antagonist. In one, the absolute risk reduction over 1 year in CV and cerebrovascular events was 18.1% (P=0.032) in favor of the antagonist.19 In the other, the GnRH antagonist relugolix was associated with a more than 50% lower relative rate of CV events (HR 0.44; 95% CI 0.24 – 0.88) even though the proportion of patients with castration-level testosterone suppression at 1 year was numerically higher on the antagonist therapy (96.7% vs. 88.8%).20
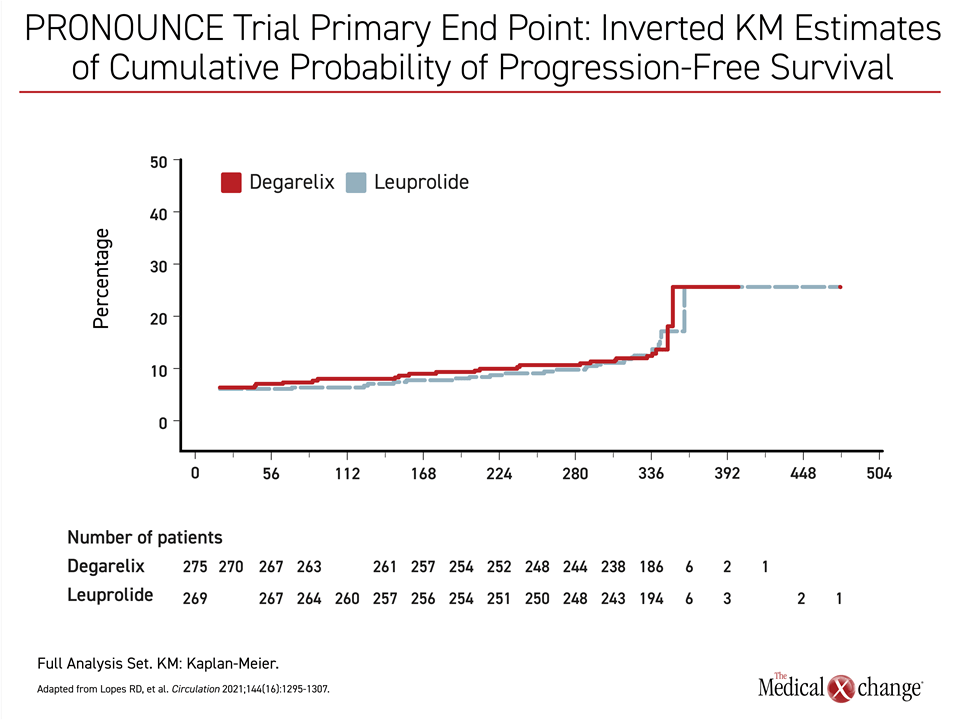
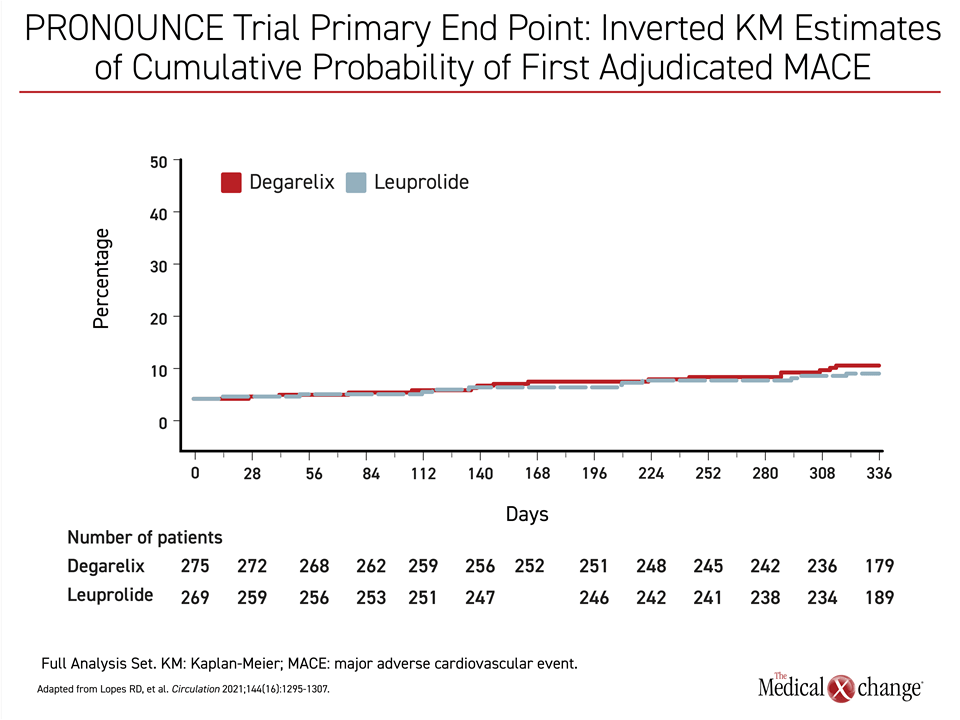
One criticism of these data is that relative CV safety has not been a prespecified endpoint for comparison in several of these trials. This provided the basis for a recently published trial called PRONOUNCE, which was designed to address this gap in the data.21 The study had not completed enrollment when it was terminated early, which occurred at the start of the COVID-19 pandemic. For the endpoint of progression-free survival (PFS), the event lines were essentially superimposable (Figure 3). Due to the low rate of events at the time of early termination when only about half of the planned enrollment had been achieved, the results with respect to CV events were inconclusive. The rate of MIs was lower in the agonist group, but more CV deaths occurred in the agonist group. These differences were not significant (Figure 4). The low rate of events prevented the central question from being addressed. This was a particular disappointment, because this contemporary study compared GnRH antagonists and agonists in a population tightly managed for CV risk. One interesting aspect of PRONOUNCE was that all enrolled patients were required to be under the care of a cardiologist and CV therapies were added as needed. This suggests that optimizing CV care in men on ADT may significantly reduce the risk of subsequent CV events and emphasizes the importance of CV screening and early management.
The mechanism of protection from CV events among patients receiving GnRH antagonists relative to agonists is unclear. There is experimental evidence, particularly in transgenic murine models, that GnRH agonists, due to their agonist mode of action, activate the GnRH receptors of T cells and macrophages embedded in endothelial plaques, resulting in plaque destabilization and rupture.22,23 This may benefit both patients with pre-existing plaques, and those who go on to develop further plaques during the course of treatment, which may be prolonged. Long term studies of this phenomenon are warranted.
In patients with prostate cancer who are candidates for ADT, CV risk assessment and medication should be initiated at the time of diagnosis. A recent US study of more than 90,000 veterans with prostate cancer revealed that almost one third did not undergo comprehensive CV risk assessment.24 Even after CV risk evaluation, more than one half had uncontrolled modifiable CV risk factors, and nearly one third with modifiable risk factors were not receiving therapy. In the subgroup of patients receiving ADT, including those with known CV disease, the rates of CV risk assessment and treatment were only modestly increased.
In the most recent CUA publications outlining strategies for addressing CV risks, baseline evaluation is recommended for all patients.1 For patients initiating ADT, the recommendations are even more explicit.4 In these guidelines, screening for diabetes, dyslipidemias, obesity, and lifestyle risks for CV disease, including smoking, unhealthy dietary practices, and inadequate exercise is recommended prior to the initiation of ADT. In patients with significant CV disease, including a history of MI or stroke, the guidelines suggest consideration of a GnRH antagonist over a GnRH agonist for risk modification. This must be balanced against the shorter duration of the antagonist depot formulation (1 month vs 3-6 months) and the increased rate of local site reactions with the antagonist.
Although additional health considerations are covered in the CUA guidelines for prostate cancer treatment with ADT, including recommendations to maintain bone health, reduce risk of anemia, and maintain an acceptable quality of life, CV events represent the most significant risk associated with ADT. CV disease represents a major threat to long-term survival in patients with prostate cancer.
Conclusion
The close association between prostate cancer and cardiometabolic health led the CUA guidelines to emphasize routine screening and monitoring of cardiac risk factors as well as the treatment of pre-existing CV disease, especially for patients being initiated on ADT. The favorable impact of GnRH antagonists and GnRH agonists on control of prostate cancer has been similar in the many settings in which ADT are indicated. GnRH antagonists appear to be underused in settings of high CV risk. Introduced later than GnRH agonists,25 GnRH antagonists have been prescribed more sparingly than GnRH agonists in Canada and elsewhere despite guidelines that suggest that GnRH antagonists offer similar efficacy. GnRH antagonists are an appropriate choice for prostate cancer patients with established CV disease and a reasonable choice for any candidate for ADT. For CV disease, which is a common cause of death in patients with early stages of prostate cancer and the most common cause of death in those who survive prostate cancer, CV safety is an underappreciated consideration when selecting among options for testosterone suppression.
The Cardiologist’s Perspective: Multidisciplinary Approach to Manage Impact of ADT on CV Outcomes in Prostate Cancer
Dr. Darryl P. Leong, MBBS (Hons) MPH M.Biostat PhD FRACP FESC
Rationale for a Collaborative Approach to Managing CV Risk and Selecting Treatment
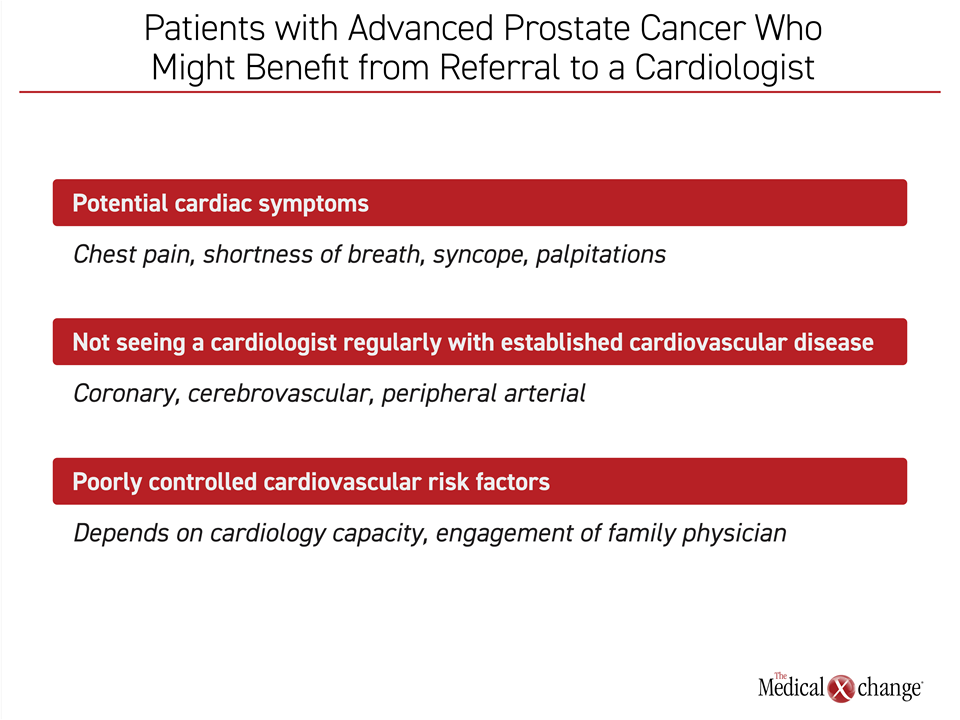
Due to the frequency with which prostate cancer and ischemic heart disease coexist in an aging male population, patients with prostate cancer should undergo comprehensive CV risk assessment followed by treatment of those risks that are modifiable. There are several basic principles. Due to the strong likelihood that men over the age of 60 have important CV risk factors or asymptomatic CV disease even in the absence of prior events, a multidisciplinary approach to risk assessment and management is appropriate. Prostate cancer is a slowly progressing malignancy: to improve survival across health risks beyond cancer, a holistic approach involving oncologists, urologists, cardiologists, and primary care physicians should be considered.
Guidelines and Evidence-based Trials Offer Insight into Treatment Goals in Clinical Practice
The rationale for the participation of a cardiologist stems from evidence that screening and early management of CV risk factors play a role in optimizing clinical outcomes. A collaborative approach is also appropriate for selecting prostate cancer therapies that influence CV risk, particularly androgen deprivation therapy (ADT).
ADT is an effective treatment at several stages of prostate cancer, but drugs within this class have variable adverse impacts on CV risk. The Canadian Urological Association has expressed a preference for GnRH antagonists over other ADT in those with pre-existing CV disease. In patients without established coronary artery disease, there are also data suggesting GnRH antagonists may pose a lower risk than GnRH agonists for adverse events related to CV disease. However, more research is needed to confirm these observations. Within a multidisciplinary collaboration, cardiologists can guide treatment to an optimal balance of benefit to risk for treatment that includes ADT.
CV Screening and Early Assessment is Key to Reach Optimal Outcomes
In a recent review, the Canadian Urological Association provided detailed descriptions of CV risk assessments. While it is helpful for oncologists to be familiar with the basic steps, a multidisciplinary approach allows each specialist to focus on his or her realm of expertise, a particular advantage in patients with a complicated clinical profile.
Almost all prostate cancer patients face other health risks beyond their malignancy, but CV disease is the most significant threat. A collaborative approach among specialists provides the best routine to optimal care (Figure 5).
References
1. Kenk M, Gregoire JC, Cote MA, et al. Optimizing screening and management of cardiovascular health in prostate cancer: A review. Can Urol Assoc J 2020;14(9):E458-E464. DOI: 10.5489/cuaj.6685.
2. Karzai FH, Madan RA, Dahut WL. Metabolic syndrome in prostate cancer: impact on risk and outcomes. Future Oncol 2016;12(16):1947-55. DOI: 10.2217/fon-2016-0061.
3. Bhindi B, Locke J, Alibhai SMH, et al. Dissecting the association between metabolic syndrome and prostate cancer risk: analysis of a large clinical cohort. Eur Urol 2015;67(1):64-70. DOI: 10.1016/j.eururo.2014.01.040.
4. Kokorovic A, So AI, Serag H, et al. Canadian Urological Association guideline on androgen deprivation therapy: Adverse events and management strategies. Can Urol Assoc J 2021;15(6):E307-E322. DOI: 10.5489/cuaj.7355.
5. Brenner DR, Weir HK, Demers AA, et al. Projected estimates of cancer in Canada in 2020. CMAJ 2020;192(9):E199-E205. DOI: 10.1503/cmaj.191292.
6. Ji G, Huang C, Song G, et al. Are the Pathological Characteristics of Prostate Cancer More Aggressive or More Indolent Depending upon the Patient Age? Biomed Res Int 2017;2017:1438027. DOI: 10.1155/2017/1438027.
7. Weiner AB, Li EV, Desai AS, Press DJ, Schaeffer EM. Cause of death during prostate cancer survivorship: A contemporary, US population-based analysis. Cancer 2021;127(16):2895-2904. DOI: 10.1002/cncr.33584.
8. Elmehrath AO, Afifi AM, Al-Husseini MJ, et al. Causes of Death Among Patients With Metastatic Prostate Cancer in the US From 2000 to 2016. JAMA Netw Open 2021;4(8):e2119568. DOI: 10.1001/jamanetworkopen.2021.19568.
9. Magee DE, Singal RK. Androgen deprivation therapy: indications, methods of utilization, side effects and their management. Can J Urol 2020;27(27 Suppl 1):11-16. (https://www.ncbi.nlm.nih.gov/pubmed/32101695).
10. Crawford ED, Heidenreich A, Lawrentschuk N, et al. Androgen-targeted therapy in men with prostate cancer: evolving practice and future considerations. Prostate Cancer Prostatic Dis 2019;22(1):24-38. DOI: 10.1038/s41391-018-0079-0.
11. Horwitz EM, Bae K, Hanks GE, et al. Ten-year follow-up of radiation therapy oncology group protocol 92-02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol 2008;26(15):2497-504. DOI: 10.1200/JCO.2007.14.9021.
12. Culig Z, Santer FR. Androgen receptor signaling in prostate cancer. Cancer Metastasis Rev 2014;33(2-3):413-27. DOI: 10.1007/s10555-013-9474-0.
13. Boccon-Gibod L, van der Meulen E, Persson BE. An update on the use of gonadotropin-releasing hormone antagonists in prostate cancer. Ther Adv Urol 2011;3(3):127-40. DOI: 10.1177/1756287211414457.
14. van Poppel H, Nilsson S. Testosterone surge: rationale for gonadotropin-releasing hormone blockers? Urology 2008;71(6):1001-6. DOI: 10.1016/j.urology.2007.12.070.
15. NCCN. Prostate Cancer. Version 32022. January 10, 2022.
16. Klotz L, Boccon-Gibod L, Shore ND, et al. The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer. BJU Int 2008;102(11):1531-8. DOI: 10.1111/j.1464-410X.2008.08183.x.
17. Keating NL, O’Malley AJ, Freedland SJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. J Natl Cancer Inst 2010;102(1):39-46. DOI: 10.1093/jnci/djp404.
18. Albertsen PC, Klotz L, Tombal B, Grady J, Olesen TK, Nilsson J. Cardiovascular morbidity associated with gonadotropin releasing hormone agonists and an antagonist. Eur Urol 2014;65(3):565-73. DOI: 10.1016/j.eururo.2013.10.032.
19. Margel D, Peer A, Ber Y, et al. Cardiovascular Morbidity in a Randomized Trial Comparing GnRH Agonist and GnRH Antagonist among Patients with Advanced Prostate Cancer and Preexisting Cardiovascular Disease. J Urol 2019;202(6):1199-1208. DOI: 10.1097/JU.0000000000000384.
20. Shore ND, Saad F, Cookson MS, et al. Oral Relugolix for Androgen-Deprivation Therapy in Advanced Prostate Cancer. N Engl J Med 2020;382(23):2187-2196. DOI: 10.1056/NEJMoa2004325.
21. Lopes RD, Higano CS, Slovin SF, et al. Cardiovascular Safety of Degarelix Versus Leuprolide in Patients With Prostate Cancer: The Primary Results of the PRONOUNCE Randomized Trial. Circulation 2021;144(16):1295-1307. DOI: 10.1161/CIRCULATIONAHA.121.056810.
22. Tivesten A, Pinthus JH, Clarke N, Duivenvoorden W, Nilsson J. Cardiovascular risk with androgen deprivation therapy for prostate cancer: potential mechanisms. Urol Oncol 2015;33(11):464-75. DOI: 10.1016/j.urolonc.2015.05.030.
23. Knutsson A, Hsiung S, Celik S, et al. Treatment with a GnRH receptor agonist, but not the GnRH receptor antagonist degarelix, induces atherosclerotic plaque instability in ApoE(-/-) mice. Sci Rep 2016;6:26220. DOI: 10.1038/srep26220.
24. Sun L, Parikh RB, Hubbard RA, et al. Assessment and Management of Cardiovascular Risk Factors Among US Veterans With Prostate Cancer. JAMA Netw Open 2021;4(2):e210070. DOI: 10.1001/jamanetworkopen.2021.0070.
25. Moul JW. Twenty-five year evolution of medical hormonal therapy for prostate cancer. BJU Int 2009;103(2):145-6. DOI: 10.1111/j.1464-410X.2008.08271.x.