Oncology
American Society of Clinical Oncology (ASCO) 2022 Annual Meeting
New Clinical Data Support Role of Gut Microbiome in Mediating Response to Immunotherapy
Chicago – A growing body of evidence suggests that the health of the gut microbiome is critical for priming the response to immune checkpoint inhibitor (ICI) therapy according to a series of recent studies, including a small but compelling clinical trial presented at this year’s ASCO Annual Meeting. The focus of this phase 1 trial was to evaluate safety when pre-treating stage 4 melanoma patients with fecal microbiota transplantation (FMT) prior to an anti-programmed cell death 1 (PD-1) ICI therapy. FMT is known to improve microbiota diversity, which is one of the key characteristics of a healthy gut microbiome. Evaluating signs of efficacy represented a secondary endpoint.
An interaction between the gut microbiome and the efficacy of PD-1-based immunotherapy is now being widely studied based on work pioneered by investigators at the Gustave Roussy Cancer Campus, Villejuif, France. Involved in these initial studies, Dr. Bertrand Routy, Scientific Director, Immunotherapy and Microbiome laboratory, University of Montreal Research Center, was also co-author of the trial presented here (Miller WH, Routy B, Jamal R, et al. Fecal microbiota transplantation followed by anti-PD-1 treatment in patients with advanced melanoma. Poster 9533).
“The gut microbiome is already established as a biomarker of response to immunotherapy in patients with melanoma,” explained another co-author, Dr. Arielle Elkrief, who worked with Dr. Routy at the University of Montreal Research Center and is now a Research Fellow at the Memorial Sloan Kettering Cancer Center, New York City, NY. She said FMT is one of the strategies being pursued to see whether the gut microbiome can be altered to improve the response to ICI treatment.
The investigation involved 20 patients with advanced melanoma who were recruited from 3 Canadian centers. All but 2 patients had stage 4 disease. In 25% of the cases, a BRAF mutation was present. The average age was 75.5 years. As part of this trial, the immunogenicity of feces from the patients before and after administration of FMT was evaluated in a murine tumor model.
This trial had no control arm, but the activity was higher than that seen historically and supported its hypothesis, reported Dr. Wilson H. Miller, Professor, Division of Experimental Medicine, McGill University, Montreal, Quebec.
Primary Safety Objective Met
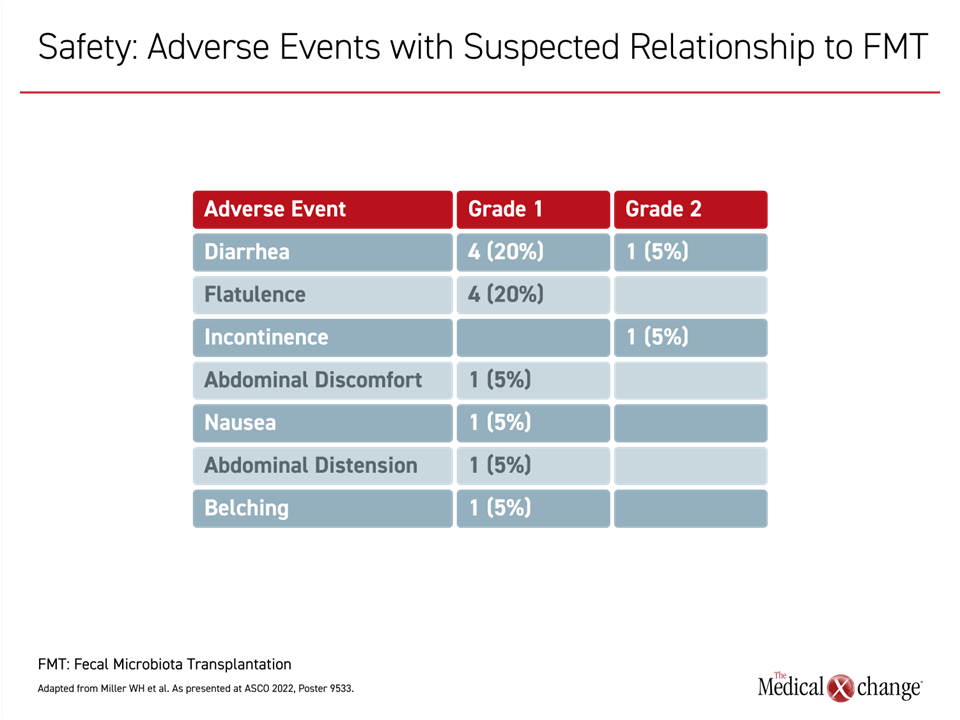
The FMT, which was taken in oral pills and chosen specifically for its microbiota diversity, was well tolerated. Although more than half of patients reported gastrointestinal adverse events (AEs), such as diarrhea or abdominal discomfort, all were of grade 1 severity. Two patients discontinued ICI therapy due to an AE, but no AE was considered serious (Figure 1).
The administration of FMT one week prior to anti-PD-1 treatment produced an objective response rate (ORR) in 13 (65%) of 20 patients. Three achieved a complete response (CR), reported Dr. Miller.
In addition to the relatively high ORR rates in a population with advanced disease, 75% had a clinical benefit characterized as stable disease or better lasting more than 6 months. Median progression-free survival in this patient population was not reached after a median of 11.2 months of follow-up.
Notably, there was an upregulation of interleukin-17 (IL-17) that correlated with increased amounts of Th17 cells in the peripheral blood following administration of FMT. This is considered a signal of increased immune system activity, which is considered critical to the benefit of ICI therapy.
The murine model supported the clinical observations. When the mouse tumors were exposed to feces from patients obtained prior to FMT, sensitivity to ICI remained low. Following FMT, sensitization was observed but only from feces obtained from patients who had a response to the ICI therapy.
Conclusion
The primary result of the trial is that FMT followed by anti-PD-1 treatment is safe. However, the activity of the ICI following FMT is consistent with a growing body of evidence that the health of the gut microbiome influences the ability of the immune system to respond to immunotherapies.
This trial was small, but the data provide “a proof of principle” that the health of the gut microbiome is important to the activity of immunotherapy, according to Dr. Miller. He said this work is “paving the way for future microbiome-based interventions” that might be important to ensuring responses to ICI or other immunotherapies.