Rheumatology
GRAPPA Annual Meeting and Trainee Symposium 2022
Advancements in Psoriatic Arthritis Management: Updated GRAPPA Treatment Recommendations
Brooklyn – New evidence-based guidelines for the management of psoriatic arthritis (PsA) have granted broader indications to newer biologic disease-modifying antirheumatic drugs (bDMARDs). Along with conventional synthetic antirheumatic drugs (csDMARDs) and targeted synthetic antirheumatic drugs (tsDMARDs), the newer bDMARDs are considered to be reasonable first-line treatments. In these revised PsA guidelines, issued by the Group of Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA), only tumour necrosis factor inhibitors (TNFi) and interleukin-17 inhibitors (IL-17i) are recommended for each of the six key domains in first-line and switch strategies. The guidelines were issued prior to the 2022 GRAPPA annual meeting, where key take-home messages were reinforced.
PsA, which the incidence and prevalence in Canada is similar to that reported in Europe and the United States, is a challenging disease not only because of its chronicity but because of the variability in disease manifestations. To address the heterogeneity, the new GRAPPA guidelines organize treatment recommendations by a six-domain approach. These domains are peripheral arthritis, axial disease, enthesitis, dactylitis, skin involvement, and nail involvement.
For an exclusive interview with Dr. Caylib Durand on the impact to clinical practice, click here.
To address these domains, the overarching principles of treatment have expanded in the revised guidelines from six to eight. The most important is to achieve the lowest possible disease activity in all six domains. Other goals include optimizing functional status and quality of life. The guidelines also emphasize the value of managing PsA with patient-reported measures of efficacy and tolerability. A comprehensive approach to patient well-being should include control of comorbidities, such as depression, cardiovascular disease, and hepatic disease.
New Versus Old Guidelines
GRAPPA issued its first PsA guidelines in 2009 with a revised version in 2015. Due to a substantial expansion in therapeutic options since the last set of guidelines were issued, a new set of recommendations was based on a thorough literature search conducted by several GRAPPA subcommittees. The final recommendations were reached on the basis of evidence and the GRADE process of assessing and debating evidence strength.
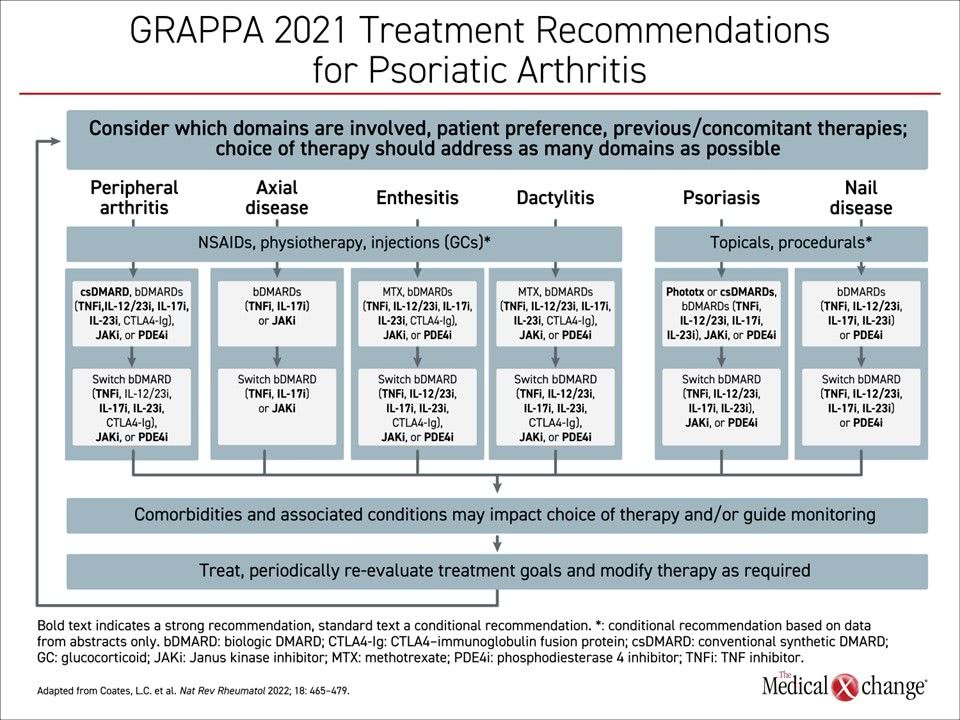
On this basis, many of the newer bDMARDs have been given strong recommendations for use in the first-line setting for multiple domains. However, only two classes of drugs received a strong recommendation for all six domains. These are TNFi and IL-17i. The strong recommendations for each are in regard to both first-line treatment and for switch or second-line therapy (Figure 1).
Although none of the newer bDMARDs besides IL-17i have been identified as appropriate across all domains, several have received strong recommendations as first-line therapy in specific domains. For example, IL-23i but not IL-12/23i, are recommended for peripheral arthritis in bDMARD-experienced patients, but neither of these biologic classes is recommended for axial domain involvement.
In the axial domain, TNFi and JAKi are also (along with nonsteroidal anti-inflammatory drugs and analgesics) strongly recommended, but the evidence is weaker. There is only one phase 3 study conducted specifically in PsA with axial manifestations to provide level 1 evidence of benefit. This study, called MAXIMISE was conducted with the IL-17i secukinumab. In the placebo-controlled trial, the odds ratio (OR) for response with either the 150 mg or 300 mg dose was about 4 times greater with the IL-17i relative to placebo (P<0.0001 for both doses).
In the peripheral arthritis domain, TNFi and IL-17i have comparable benefit for control of joint symptoms, but IL-17i is more active against skin involvement, suggesting this biologic is a particularly good choice in those patients when psoriatic lesions are present.
Due to the frequency with which patients with PsA have disease activity in multiple domains, including all six domains, the new GRAPPA recommendations encourage selection of therapies that are broadly effective across all domains.
The relative roles of drugs within the different classes of medications reflects an emphasis on individualized treatment. Across domains, available agents even within a general class, such as bDMARDs or tsDMARDs, should not be considered interchangeable. Due to the frequency with which patients with PsA have disease activity in multiple domains, including all six domains, the new GRAPPA recommendations encourage selection of therapies that are broadly effective across all domains.
The GRAPPA recommendations have also provided revised recommendations on other aspects of patient management, such as when and how to taper treatments and when to consider biosimilars, but the updated organization of “strongly” recommended therapies is perhaps the greatest departure from the previous set of guidelines.
Summary
The updated GRAPPA guidelines, like the previous set, have assumed a domain-based approach to the selection of treatments. Based on current evidence, these guidelines acknowledge that the most advanced options are not interchangeable across each of the domains of disease. The only therapies strongly recommended for all 6 domains are TNFi and IL-17i. For the axial domain, IL-17i is the only option supported by level 1 evidence.