Oncology
European Society of Medical Oncology (ESMO) Congress 2022
Gaining Further Perspective into Treatment of Advanced Breast Cancer with CDK4/6 Inhibitors
Paris – In the first-line therapy for hormone receptor-positive, human epidermal growth factor receptor 2-negative (HR+/HER2-) advanced breast cancer in postmenopausal women, overall survival (OS) data have been presented for the last of 3 major multicenter randomized trials to evaluate a CDK4/6 inhibitor in this population. This latest trial, called MONARCH 3, was presented at ESMO and associated a CDK4/6 inhibitor with a favourable trend in the interim analysis but not a statistically significant advantage over hormone therapy alone. These results differ from previous OS data from the other two trials, MONALEESA-2 demonstrating a significant advantage and PALOMA-2 showing no signal of a benefit, despite similar designs, entry criteria, and benefit for the outcome of progression-free survival (PFS). For OS, CDK4/6 inhibitors do not appear to be interchangeable.
In the MONARCH 3 trial, the comparison was between the addition of abemaciclib or placebo to one of two aromatase inhibitors (anastrozole or letrozole). In a trial presented 1 year ago, MONALEESA-2, the addition of ribociclib was compared to letrozole alone. The addition of palbociclib to letrozole alone was evaluated in PALOMA-2. Relative to the highly significant OS benefit shown with ribociclib in MONALEESA-2 and the lack of a statistically significant OS benefit in PALOMA-2, the MONARCH 3 trial showed a favourable trend that has not yet reached statistical significance
For an exclusive interview with Dr. Karen Gelmon on the impact to clinical practice, click here
Results show that CDK4/6 Inhibitors are not Interchangeable
“These are not identical compounds. They have different pharmacodynamic profiles, different dosages, and they produced different results in comparable previously conducted adjuvant treatment trials,” said Dr. Meritxell Bellet Ezquerra, Senior Researcher, Vall d’Hebron Institute of Oncology, Barcelona, Spain. An expert in metastatic breast cancer, Dr. Bellet Ezquerra was invited by ESMO to place the late-breaking results of MONARCH 3 into clinical context.
“These are not identical compounds. They have different pharmacodynamic profiles, different dosages, and they produced different results in comparable trials.”
Dr. Bellet Ezquerra emphasized that the MONARCH 3 OS results were analyzed as an interim analysis of ongoing follow-up and are not negative. Rather, based on the trajectory of the OS, she considers an OS benefit to be “likely” at a final analysis, which is expected sometime in 2023. All three of the CDK4/6 inhibitors demonstrated favourable PFS data, however, the OS data should guide the current choice of CDK4/6 inhibitors, according to Dr. Bellet Ezquerra.
“Ribociclib has the maximum level of evidence of OS in the first-line setting and so it should be considered first,” she said. However, she did not rule out either abemaciclib or even palbociclib as reasonable choices if there was a compelling rationale to consider an alternative, such as contraindications.
“[The CDK4/6 inhibitor with] the maximum level of evidence of OS in the first-line setting should be considered first.”
CDK4/6 Inhibitor Trials Challenge Standard of Care
In MONARCH 3, 493 postmenopausal women with HR+/HER2- advanced breast cancer were randomized in a 2:1 ratio to the experimental or control arms. In the experimental arm, patients received 150 mg abemaciclib twice daily plus either 1 mg of anastrozole or 2.5 mg of letrozole once daily. In the control arm, patients received either of the aromatase inhibitors alone. At the time the MONARCH 3 trial was initiated, as with the MONALEESA-2 and PALOMA-2 trials, hormone therapy was still the standard of care.
The primary endpoint of PFS was reported in 2017. At that time, the median PFS was not reached for the experimental arm versus 14.7 months for hormone therapy alone, translating into a highly significant 46% improvement in the risk of death or progression (HR 0.54; P=0.000021). Similar PFS benefits were associated with the other CDK4/6 trials. In 2016, the PFS data showed exactly the same relative improvement in PFS for ribociclib versus hormone therapy alone (not reached vs. 14.7 months). The PFS data for palbociclib in PALOMA-2 were also published in 2016, associating the CDK4/6 inhibitor with a median PFS of 24.8 months versus 14.5 months for hormone therapy alone.
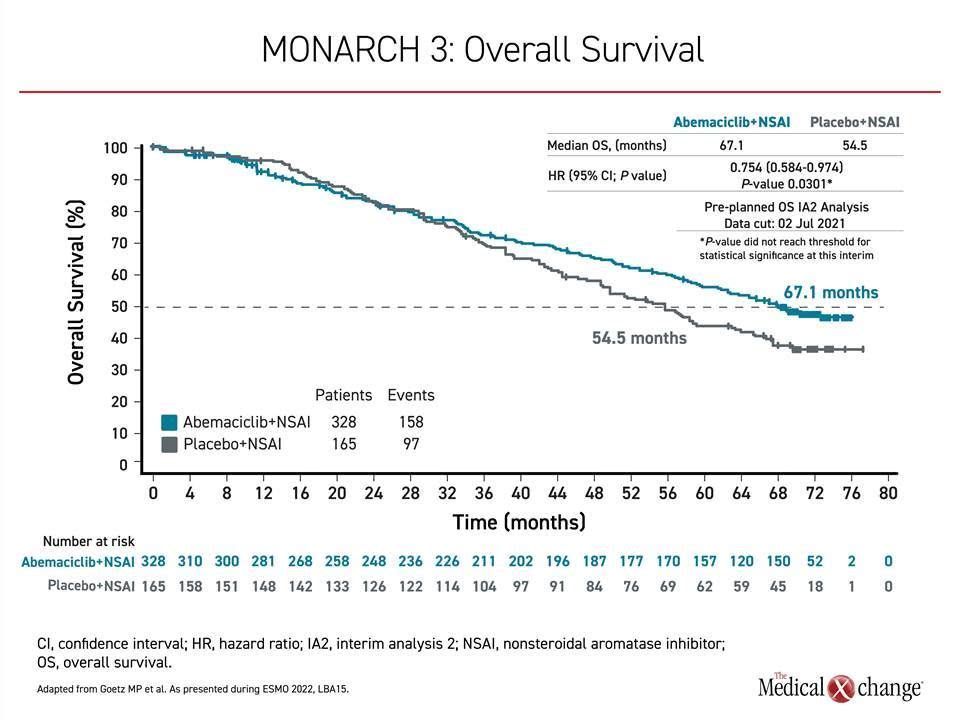
To assess OS benefit in MONARCH 3, two interim and a final analysis were planned. The first interim analysis, after 189 deaths, was conducted in 2020 and did not associate abemaciclib with a significant advantage. In the current second analysis, conducted after 252 deaths and a mean of 70.2 months of follow-up, the median OS was 67.1 months on the CDK4/6 inhibitor versus 54.5 months in the control group. The hazard ratio corresponded with about a 26% reduction in risk of death (HR 0.0754; P=0.0301), but the predefined boundary of OS benefit by alpha computation was not crossed (Figure 1).
As a result, “statistical significance was not reached,” reported the principal investigator, Dr. Matthew Goetz, Department of Oncology, Mayo Clinic, Rochester, Minnesota.
However, the data “are moving favourably,” he said. The median increase in OS was just over 12 months, and there were additional signs of benefit. In particular, the OS improvement in the subgroup of patients with visceral disease at baseline was more than 16 months (48.8 vs. 65.1 months), producing about a 30% reduction in risk of death in this group (HR 0.708; P=0.0392). Again, statistical significance was not reached, but, like total OS, the difference between the survival curves of those randomized to the CDK4/6 inhibitor versus the controls appears to be still widening at the most recent analysis.
Although statistical significance “was not reached” for an OS benefit in the MONARCH 3 trial, the data “are moving in a favourable direction.”
Survival Curves Separated at 3 Years
Survival curves for the experimental and control arms began to separate at about 3 years in favour of the CDK4/6 inhibitor. The advantage has continued to grow over time even though 31.5% of those randomized to the control arm, versus only 10.1% of the active treatment arm, received a subsequent CDK4/6 inhibitor. A comprehensive analysis of other subgroups, such as those separated by age, race, progesterone hormone status, geographic region, prior exposure to hormone or other therapies, and number of involved organs have also favoured the CDK4/6 inhibitor over hormone therapy alone.
In MONARCH 3, abemaciclib was also associated with about a 16-month increase in chemotherapy-free survival (46.7 vs. 30.6 months), which translated into a nearly 40% reduction, although no P value was calculated (HR 0.636).
The safety and tolerability of abemaciclib in MONARCH 3 were consistent with that reported when the PFS results were published 5 years ago. This is consistent with the safety data of the other CDK4/6 inhibitors evaluated in postmenopausal advanced breast cancer, which are high in general but not identical. In one network meta-analysis of 8 trials, discontinuation for adverse events was higher on abemaciclib than ribociclib or palbociclib, a difference attributed to more gastrointestinal adverse events (Desnoyers A et al. Cancer Treat Rev. 2020;90:102086. At the most recent analysis of MONARCH 3, there were only three adverse events of grade ≥3 severity seen more common on abemaciclib than on the aromatase inhibitor alone. These were neutropenia (27.2% vs. 1.2%), anemia (8.9% vs. 1.2%), and diarrhea (9.8 % vs. 1.2%).
OS Benefit in MONARCH 3 Appears Likely
Based on the trajectories of OS at this second interim analysis, a relative OS benefit for abemaciclib is expected in the final analysis, according to both Dr. Goetz and Dr. Bellet Ezquerra, with the latter suggesting that it is instructive and clinically important to consider the potential differences between the tested CDK4/6 inhibitors. She suggested that the outcomes support the premise that the drugs are not interchangeable for the specific indication that was the focus of the MONARCH 3, MONALEESA-2, and PALOMA-2 trials even if there were some modest differences in design and entry criteria.
PALOMA-2: Improvement in Outcome is Limited to PFS
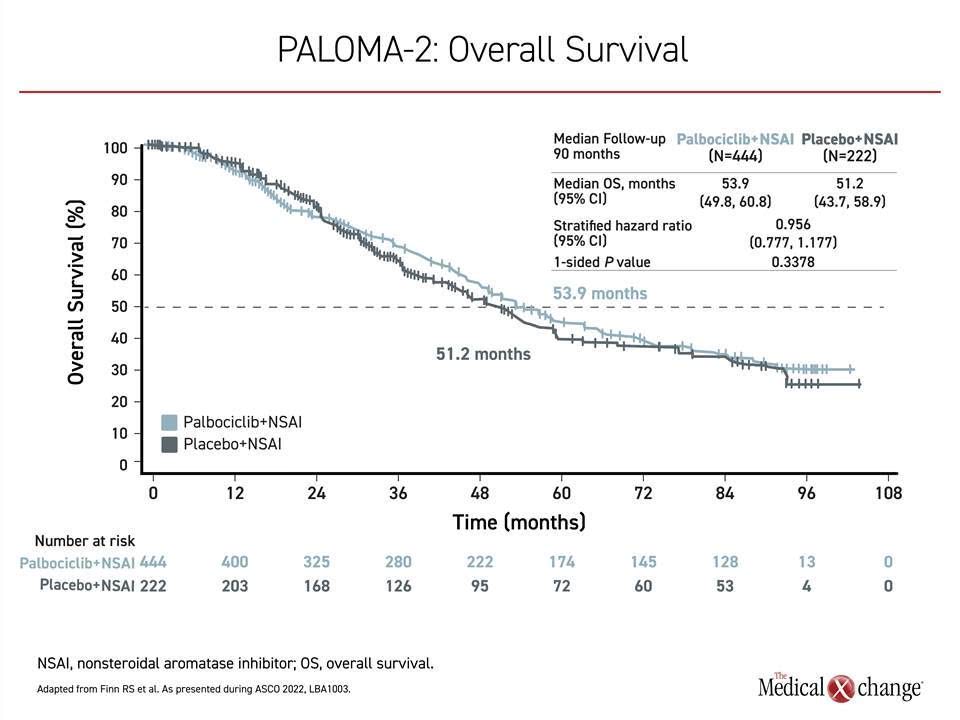
Of these trials, PALOMA-2 appears to be the most different from the other two. In this study of 666 patients randomized in a 2:1 ratio to the experimental and control arms, there was essentially no signal of an OS benefit after a median follow-up of 90 months when 405 deaths had occurred. At that time, the median OS was 53.9 months in the arm receiving palbociclib versus 51.2 months in those receiving hormone therapy alone. The 4% reduction in risk of death did not approach statistical significance (HR 0.956; P=0.3378) (Figure 2).. A post-hoc sensitivity analysis excluding those who were lost to follow-up or withdrew consent did not alter the conclusions.
Large and Significant OS Benefit in MONALEESA-2
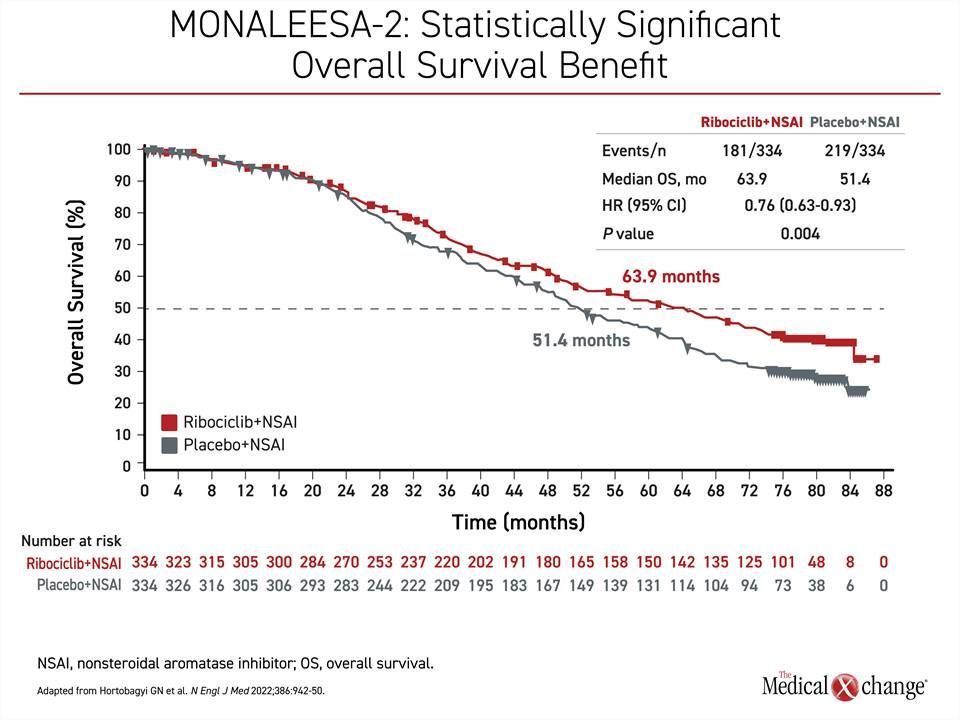
In contrast, the MONALEESA-2 trial, which randomized 668 patients in a 1:1 ratio to ribociclib plus letrozole or letrozole plus placebo, showed a large OS benefit after 80 months of follow-up when 400 deaths had occurred. The more than 12-month OS advantage (63.9 vs. 51.4 months) translated into a 24% reduction and highly statistically significant in reduction of death (HR 0.76; P=0.008) (Figure 3). As in MONARCH 3, the separation of the survival curves began at about 2 years.
In addition, the OS benefit in MONALEESA-2 is consistent with two other trials, MONALEESA-3, which also enrolled postmenopausal patients, and MONALEESA-7, which enrolled premenopausal patients, in that there was about a 30% reduction in risk of death regardless of menopausal status, with the addition of ribociclib relative to endocrine therapy alone.
A Closer Look at Three CDK4/6 Inhibitor Studies
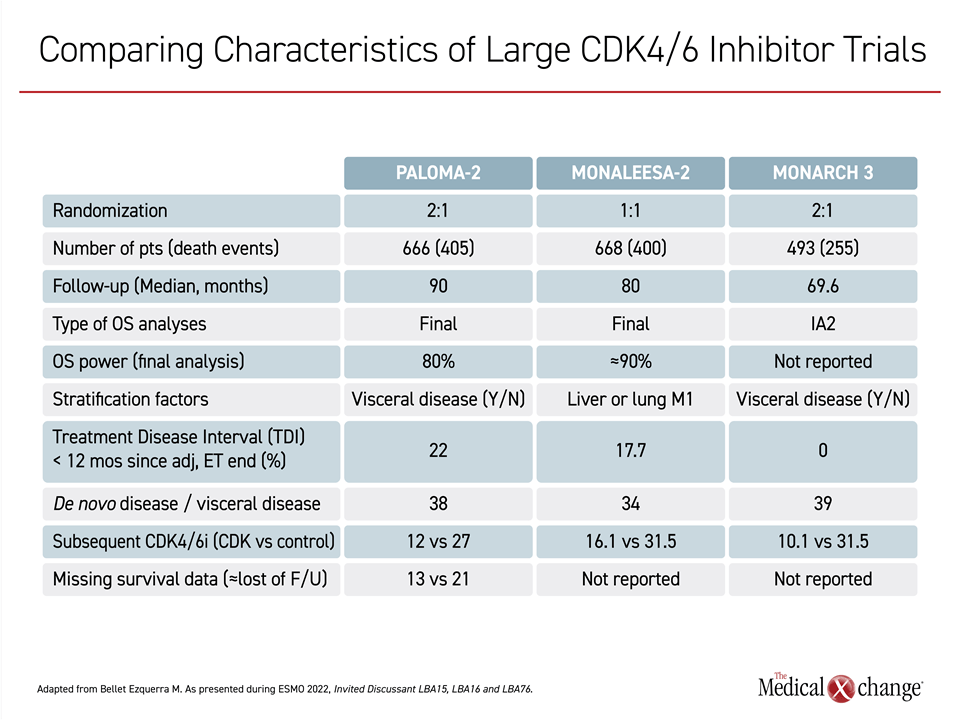
In a side-by-side comparison of potentially relevant characteristics of the three CDK4/6 inhibitor trials, Dr. Bellet Ezquerra did not find any major differences that would fully account for the differences in outcomes (Table 1).
For example, the proportion of patients receiving a subsequent CDK4/6 inhibitor in the control arm (about 30% in all 3 studies) and the proportion with de novo disease (about 35% to 40% in all 3 studies) were similar. A higher proportion of patients in PALOMA-2 (22%) entered the trial less than 12 months after the discontinuation of endocrine therapy relative to MONARCH 3 (0%) and MONALEESA-2 (17.7%), but Dr. Bellet Ezquerra did not consider this to have had a major impact on outcomes.
Despite these results, all the CDK4/6 inhibitors are likely to continue to have a role in the control of breast cancer and diseases, according to Dr. Bellet Ezquerra. Even in postmenopausal HR+/HER2- metastatic breast cancer, she said palbociclib should “remain in the equation,” based on some real-world evidence of a potential OS benefit. However, she said that available data define the current order of treatments to consider for this indication while waiting for head-to-head comparisons.
Due to the fact that only one of these drugs now has 1A level of evidence for a standard of care on the basis of OS data, ribociclib is “mostly the first option” at the current time, she said.
Conclusion
More than 7 years after the initiation of three definitive multinational trials evaluating the benefit of adding a CDK4/6 inhibitor to hormone therapy, OS outcomes are now available. Benefits varied; although a large and statistically significant OS benefit was associated with ribociclib in MONALEESA-2, no OS benefit was observed with palbociclib in PALOMA-2. The last trial to release OS data, MONARCH 3, showed a highly favourable trend for OS survival that has not yet reached statistical significance.