Cardiology
American Heart Association (AHA) 2022 Scientific Sessions
Lipid-lowering Efficacy Sustained Over 4 Years with Twice-yearly Injections of a First-in-class Therapy
Chicago – The reductions in low-density lipoprotein cholesterol (LDL-C) observed on a first-in-class small interfering RNA (siRNA) therapy remain unchanged over 4 years of therapy, according to a newly-reported extension trial, ORION-3. The safety and tolerability profile over this time has also remained unchanged. For patients who require indefinite or lifetime LDL-C control, the long-term data may change the standard of care. The data show that high-risk patients, such as those with familial hypercholesterolemia or those with prior cardiovascular events on maximally tolerated doses of statins, can expect stable reductions in LDL-C independent of most variables that threaten long-term lipid lowering.
PCSK9 inhibitor monoclonal antibodies (mAbs) altered the concept of optimal lipid control by demonstrating even greater protection against cardiovascular events in high-risk patients when LDL-C levels are reduced below those typically achieved with statins. The more recently approved siRNA therapeutic inclisiran has mimicked these LDL-C reductions but with a very long duration of action, reducing the burden of adherence and circumventing other threats to long-term efficacy, such as failure to adhere to lifestyle modifications. Unlike the 2- or 4-week dosing intervals of PCSK9 inhibitor mAbs, inclisiran is delivered on an every-6-month basis.
For an exclusive interview with Dr. Lawrence Leiter on the impact to clinical practice, click here
No Escape from LDL-C Control
No fluctuation in LDL-C reductions was seen over time in ORION-3, an extension of ORION-1, which was a phase 2 trial published more than 5 years ago (Ray KK et al. N Engl J Med 2017;376:1430-1440). “With up to 5 years of total exposure, there was no escape at all,” reported Dr. Kausik K. Ray, Director of the Centre for Cardiovascular Disease Prevention, Imperial College, London, UK. By avoiding more frequent dosing intervals, the inclisiran long-term data suggest “we can now consider annualized control of LDL,” said Dr. Ray, referring to the concept of maintaining control of LDL-C from year to year rather than from dose to dose.
With a highly-effective treatment administered just twice per year, “we can now consider annualized control of LDL.”
Almost all the patients randomized to inclisiran in ORION-1 and who then completed the trial were transitioned to the extension study. All were then maintained on a subcutaneous injection of 300 mg inclisiran sodium every 6 months during ORION-3.
Of the 127 patients randomized to placebo in ORION-1, 92 enrolled in ORION-3. This group was not initially transitioned to inclisiran. Rather, they were started on the PCSK9 inhibitor mAb evolocumab at a dose of 140 mg every 2 weeks. At the end of 1 year, 89 were transitioned to inclisiran and maintained on 300 mg for the remainder of ORION-3. Of these, 61 underwent a sequential transition to inclisiran, meaning that evolocumab was stopped before inclisiran was started, and 28 underwent a concurrent switch, meaning the first dose of inclisiran was administered the same day as the last dose of evolocumab.
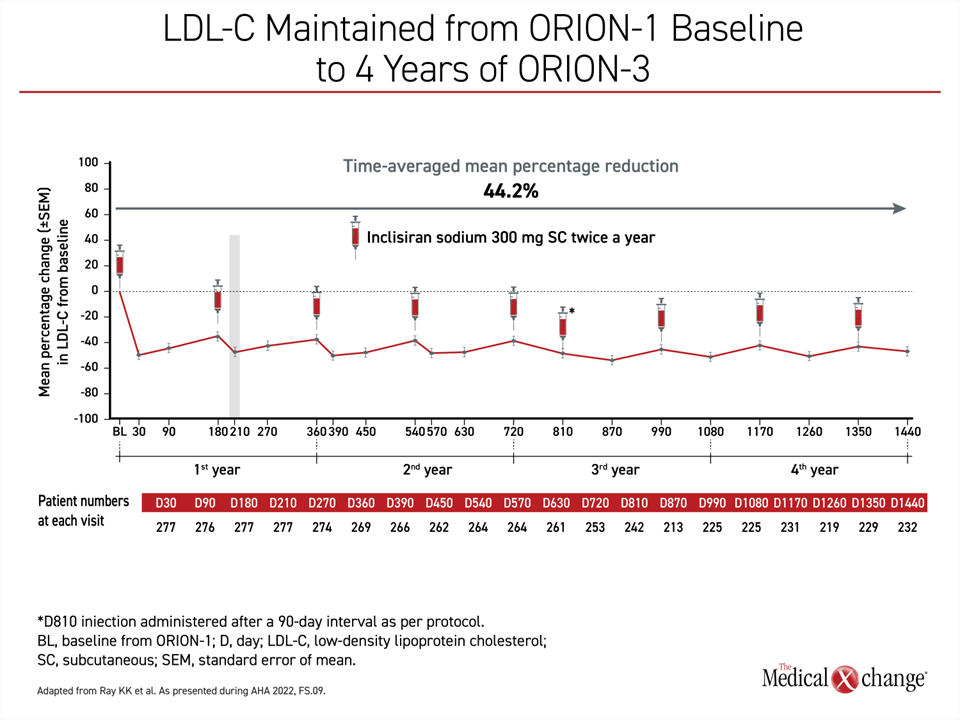
Time Averaged LDL-C Reduction was 44.2%
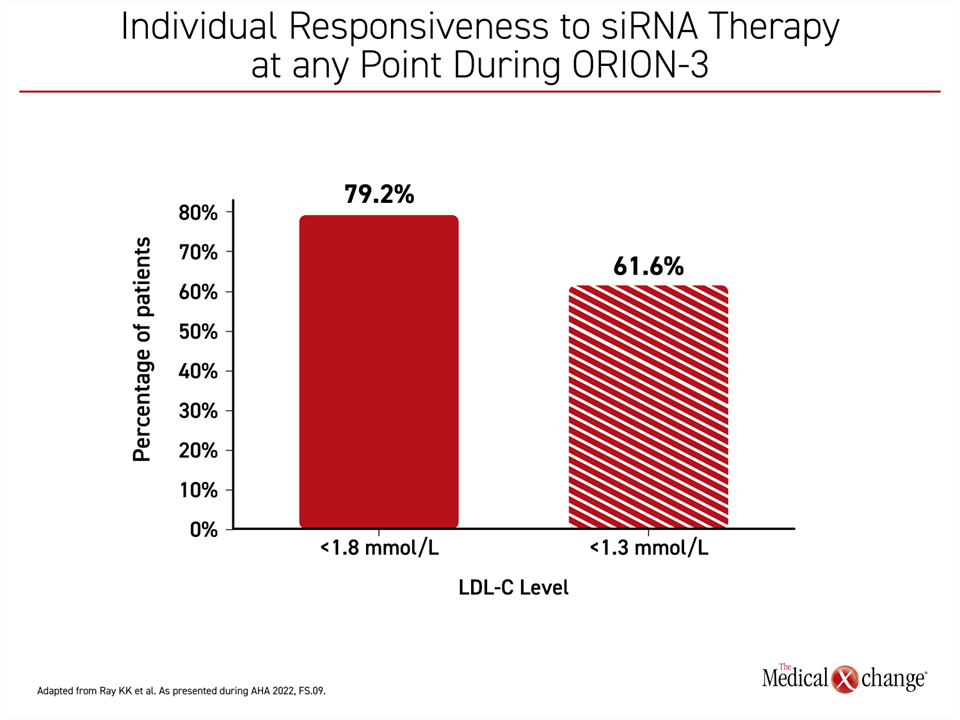
For those who remained on continuous inclisiran from ORION-1 through ORION-3, there was essentially no fluctuation in LDL-C control over the entire period of follow-up (Figure 1). The pattern of LDL-C over this period included a small rise in LDL-C prior to each of the doses at 6 months, but the nadir in the LDL-C reduction was about the same as that observed after the first dose in ORION-1. Overall, the time-averaged mean percentage reduction in LDL-C over the course of ORION-3 was 44.2%. Nearly 80% achieved an LDL-C <1.8 mmol/L (Figure 2).
At entry to ORION-3, patients initially treated with placebo in ORION-1 achieved a steep reduction in LDL-C with the PCSK9 inhibitor mAb that was maintained over the first year on this therapy. When transitioned to inclisiran at the beginning of year 2, there was a rise in LDL-C among those who stopped evolocumab before starting inclisiran, but the overall suppression of LDL-C was sustained on inclisiran whether the transition to inclisiran was sequential or concurrent. Again, the relative control of LDL-C for the remainder of ORION-3 on inclisiran was unchanged over time. The time-averaged mean reduction in this group over 4 years was 45.3%.
Placebo-like Safety Profile Unchanged Over Time
In the ORION-1 trial, the most common adverse event was injection site reactions. The most common adverse events of other types were non-specific and included myalgia, headache, fatigue, nasopharyngitis, back pain, hypertension, diarrhea, and dizziness, but none of these differed significantly between inclisiran and placebo. In ORION-3, the safety profile remained unchanged. The most common adverse event involved injection site complaints. Again, adverse events observed in more than 10% of patients were non-specific and included nasopharyngitis (19%), hypertension (14.8%), arthralgia (14.1%), urinary tract infection (13.0%), influenza (12.7%) and diabetes mellitus (11.3%). Inclisiran was discontinued due to an adverse event in 4.2% of patients.
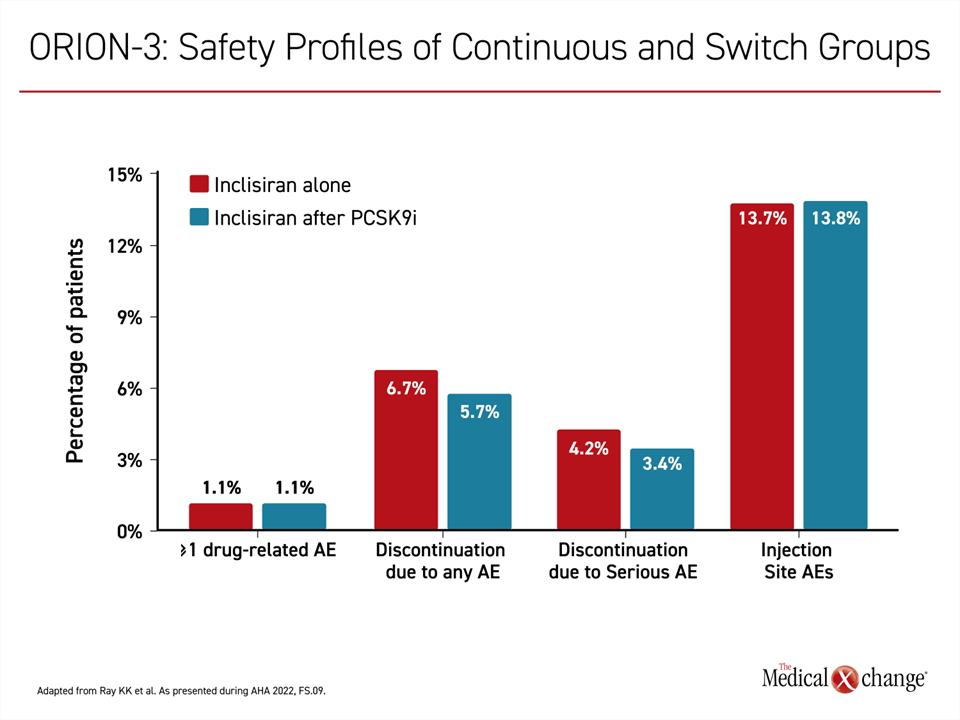
“In evaluating the adverse events over the long-term follow-up, there was really nothing to see,” Dr. Ray said. He added that there were no safety issues associated with the switch from the PCSK9 inhibitor mAb to inclisiran performed at 1 year whether performed sequentially or concurrently (Figure 3).
PCSK9 Levels Consistently Suppressed for 4 Years
In ORION-3, the impact of inclisiran on PCSK9 levels was monitored throughout follow-up. Over time, these levels remained consistently suppressed in ranges from approximately 62% to 77%. The mean percentage change from baseline at year 4 was 69.5%. In this and seen in other clinical studies, the reduction in PCSK9 and LDL-C correlated, reinforcing the mechanism of action and confirming that the target is consistently suppressed, and the clinical effect maintained over indefinite periods of chronic dosing.
“What the ORION-3 data now demonstrate is that this RNA-based mechanism can be used for biologic targets with a high degree of efficacy and safety,” Dr. Ray said.
“What the ORION-3 data now demonstrate is that this RNA-based mechanism can be used for biologic targets with a high degree of efficacy and safety.”
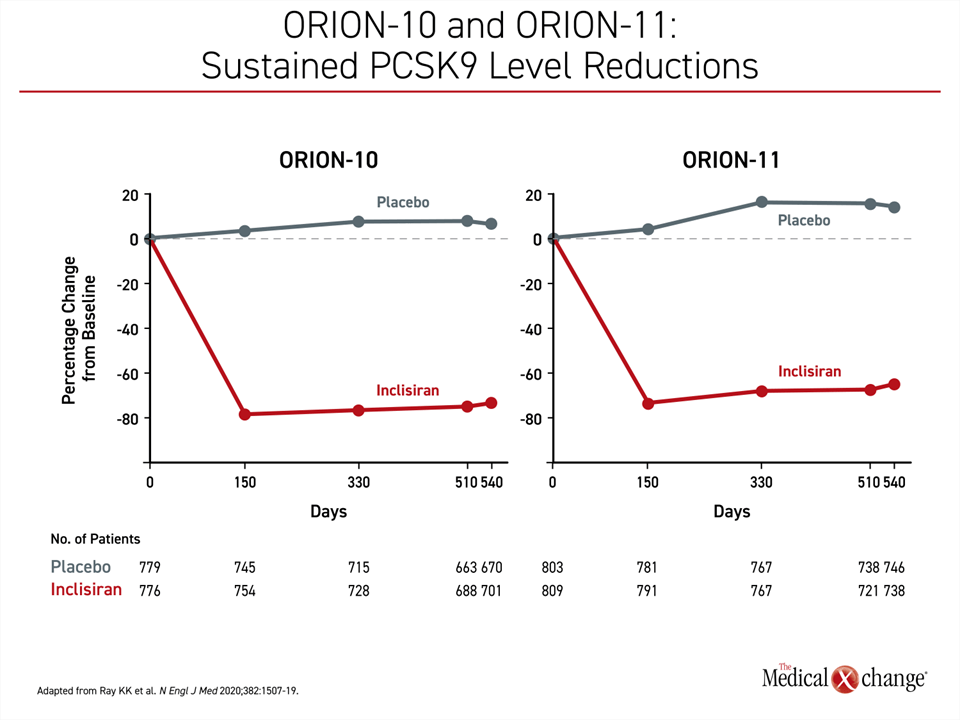
The LDL-C lowering efficacy of the siRNA inclisiran was proven in ORION-1, but the utility of the drug and regulatory approval was confirmed by the data generated by the phase 3 ORION-10 and ORION-11 trials. These trials, published together 3 years after ORION-1 (Ray KK et al. N Engl J Med 2020;382:1507-1519), each randomized approximately 1500 patients to inclisiran or placebo by subcutaneous injection. The coprimary endpoints for each trial were the placebo-corrected change in LDL-C at 510 days relative to baseline and the time-adjusted percentage change in LDL-C from baseline after day 90 and up to day 540.
The trials had the same designs, but enrollment in ORION-10, which was conducted in the US, was restricted to patients with established atherosclerotic cardiovascular disease. Patients in ORION-11, which was conducted in Europe and South Africa, were eligible if they had established cardiovascular disease or an atherosclerotic disease equivalent, such as diabetes mellitus. In both studies, patients had to have LDL-C above guideline targets despite maximally tolerated doses of statins.
Phase 3 LDL-C Reductions Unchanged Over Time
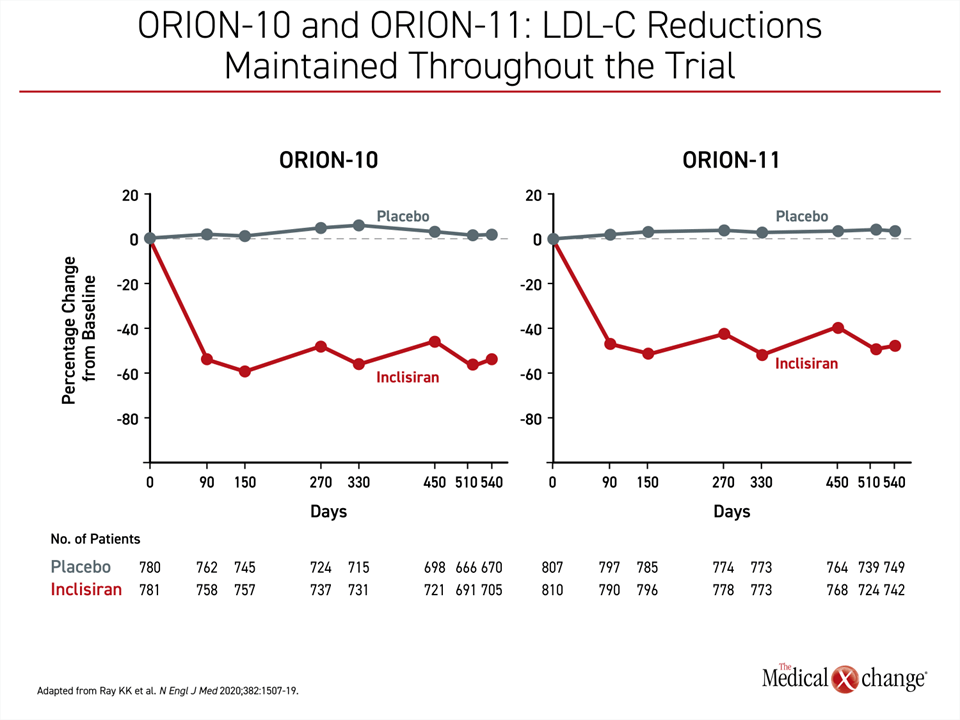
When first measured at 90 days, the LDL-C reductions in the two studies were nearly identical (Figure 4). These levels were sustained without substantial change until the end of the study. At day 510, the mean reductions in LDL-C relative to placebo were 52.3% and 49.9% in the ORION-10 and ORION-11 trials, respectively. The reductions in PCSK9 levels relative to placebo were 69.8% and 63.6%, respectively (Figure 5). There were no meaningful differences across a wide range of prespecified subgroups defined by sex, age, race, body mass index (BMI), presence or absence of metabolic diseases, such as diabetes, or renal function.
The efficacy and safety of inclisiran in the phase 3 trials provided the basis for approval of this therapy, but Dr. Ray emphasized that the longer-term data of ORION-3 have been important for demonstrating prolonged efficacy and safety for a drug that may be reasonably considered to be a lifetime therapy.
Longest Follow-up to Date Shows Consistent Safety-benefit Profile
“This is the first prospective long-term study to assess repeat hepatic exposure to any siRNA therapeutic over 5 years of total exposure,” Dr. Ray said. The evidence of sustained benefit and safety is compelling.
“This is the first prospective long-term study to assess repeat hepatic exposure to any siRNA therapeutic over 5 years of total exposure.”
“By extending the period of twice-yearly inclisiran maintenance regimen from the original 1-year window to a further 4 years, it is apparent that there is no change in the biologic response to the mechanism of LDL-C control,” Dr. Ray said. In practical terms, the ORION-1 study also supports the concept that switching from a PCSK9 inhibitor mAb to a siRNA therapeutic is safe and effective.
“With a further 4 years of follow-up, it is apparent that there is no change in the biologic response to the mechanism of LDL-C control.”
This is important data for a substantial proportion of patients who are unable to reach levels of LDL-C now understood to further reduce cardiovascular risk relative to those typically achieved on statins. In the era prior to the introduction of the more potent lipid-lowering therapies that target PCSK9, guidelines called for a relatively modest treatment target. These were based on the statin trials, where even high-intensity agents in maximally tolerated doses did not consistently achieve reductions below 1.4 mmol/L. It is now understood that patients with levels this low but with progressive atherosclerosis and recurrent events can achieve further risk reductions with much lower LDL-C targets. This is the role of agents that target the PCSK9 protein. The extended ORION-3 data show that reductions of this degree can be achieved and sustained with a siRNA therapeutic.
“The main conclusion of ORION-3 is that twice-yearly inclisiran is a convenient, well-tolerated, and effective long-term add-on lipid-lowering therapy for patients who require additional LDL-C lowering from levels that can be achieved on a statin,” Dr. Ray said.
Conclusion
The 4-year ORION-3 extension trial is significant for its demonstration that the effect of a siRNA therapy for LDL-C reduction is sustained and well tolerated for up to 4 years. It further shows its value in defining a viable treatment strategy for the substantial proportion of patients who have a high residual risk of cardiovascular events in the absence of drugs that inhibit PCSK9. In these patients, who require lifetime therapy, maximal control with therapy that has a low adherence burden will be regarded as a significant clinical advance by a substantial proportion of high-risk patients.