Cardiology
Heart Failure: Expert Review and Commentary from Published Literature
Opportunities to Improve Survival in Heart Failure in Canada
Nathaniel Hawkins, MBChB, MD, MPH
Clinical Associate Professor, University of British Columbia (UBC)
UBC Centre for Cardiovascular Innovation
Vancouver, British Columbia
Eileen O’Meara, MD, FRCPC
Cardiologist, Montreal Heart Institute
Professor of Medicine, University of Montreal
Montreal, Quebec
There are four guideline-directed medical therapies (GDMT) associated with a survival benefit in heart failure with reduced ejection fraction (HFrEF). Most HFrEF patients are eligible for all four, according to a recently published 2023 analysis from the Canadian Heart Failure registry (CAN-HF)1. The fact that a substantial proportion of eligible HFrEF patients are not receiving one or more of the foundational GDMT is a persistent source of avoidable deaths in Canada. Of the four GDMTs, the angiotensin receptor neprilysin inhibitor (ARNI) was identified as the most commonly omitted, but it was not alone. Each of the four foundational therapies—beta blockers (BB), mineralocorticoid antagonists (MRA), ARNI, and sodium-glucose cotransporter-2 inhibitors (SGLT2i)— provide substantial morbidity and mortality benefits independent of the other three. All four are required for optimal risk reductions.
Background
Landmark randomized trials over the past 30 years have established survival benefits for four classes of medical therapies in heart failure with a left ventricular ejection fraction (LVEF) ≤40%. GDMT as it is defined in the most recent guidelines from the Canadian Cardiovascular Society (CCS) is based on these trials.2 Of the current classes available to improve HFrEF survival, the benefits of three—ACEi, BB, and MRA—were established in the late 1980s up to the early 2000s. Two more classes have been added more recently; ARNI, which has superseded ACEi in the current GDMT, and SGLT2i. Thus the total number of foundational agents remains at four.
For an exclusive interview with Dr. Nathaniel Hawkins on the impact to clinical practice, click here
The first of the foundational classes of therapy were ACEi, which were shown in landmark trials to provide survival benefit relative to such standard therapies such as vasodilators and diuretics.3,4 BB joined ACEi as foundational GDMT when they provided a survival benefit relative to placebo in patients on standard therapy plus ACEi.5 Similarly, MRA, the third class, was associated with survival benefits on top of ACEi and BB.6 Angiotensin receptor blockers (ARB) were subsequently established as acceptable substitutes for ACEi in patients with ACEi intolerance or other relative contraindication for ACEi therapy.7 In many guidelines, including those issued by the CCS, these two types of drugs are often grouped together as renin-angiotensin system inhibitors (RASi).
In 2017, the CCS and other heart failure guidelines were updated to include ARNI when a survival benefit was observed with the addition of this agent to the previously established foundational GDMT therapies.8 This was due to the pivotal 2014 trial, PARADIGM-HF, where the advantage of the ARNI was demonstrated against ACEi.9 The ARNI sacubitril/valsartan contains the neprilysin inhibitor sacubitril, which inhibits activation of neurohormonal processes that contribute to cardiac remodeling, and the ARB valsartan, which like ACEi, is also from the RASi class therapies.10 In PARADIGM-HF, the survival benefit of the ARNI sacubitril/valsartan was observed over the ACEi enalapril in patients with HFrEF on the established GDMT of BB and MRA.
The GDMT changed again in the most recent CCS guidelines on the basis of trials with SGLT2i therapies.2 In both the DAPA-HF trial and the EMPEROR-Reduced trial, which were conducted with dapagliflozin and empagliflozin, respectively,11,12 the addition of an SGLT2i produced a survival benefit and reduced HF hospitalizations relative to placebo in patients already on three-class GDMT (BB, MRA, ARNI or RASi). SGLT2i, previously shown to prevent heart failure in patients with diabetes,13 have proven versatile for the control of heart failure in that subsequent trials have now supported clinical benefits from these agents even in patients in heart failure with LVEF >40%.14
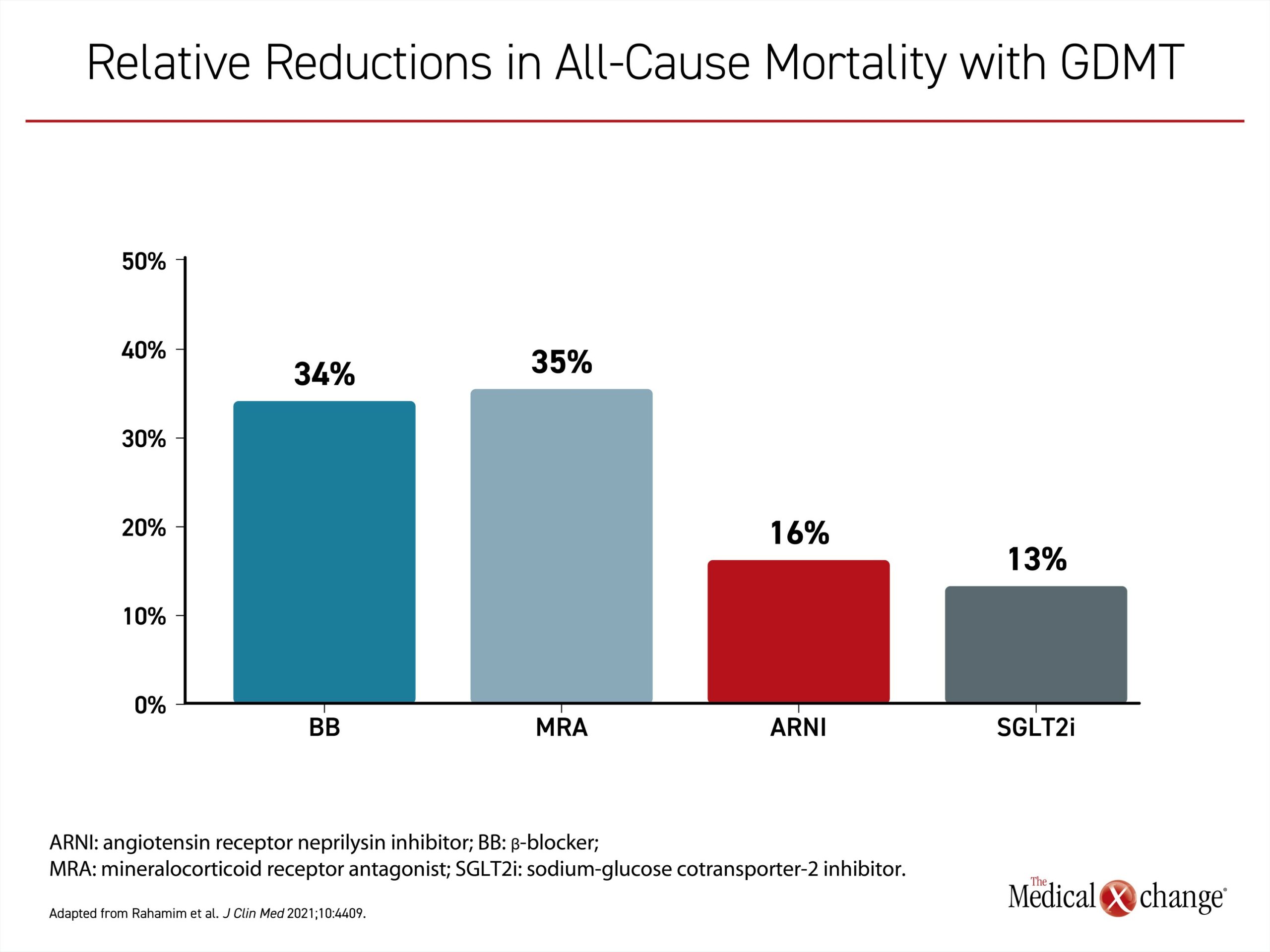
The concept of additive benefit is central to the goal of optimal mortality and morbidity reductions. All of the landmark clinical trials mandated optimal background medical therapy for that era. Each time the GDMT has been expanded, one or more major clinical trials have demonstrated a mortality benefit for the added agent on top of the previous GDMT. These benefits ranged from approximately 15% to 30% over that of the prior GDMT (Figure 1).15 Except in the small proportion of patients who are not candidates for specific therapies in the GDMT, the greatest opportunity for prolonging life in a patient with HFrEF is achieved when all agents are employed at the same time. None should be omitted and all should be initiated as quickly as possible in those with a diagnosis of HFrEF.
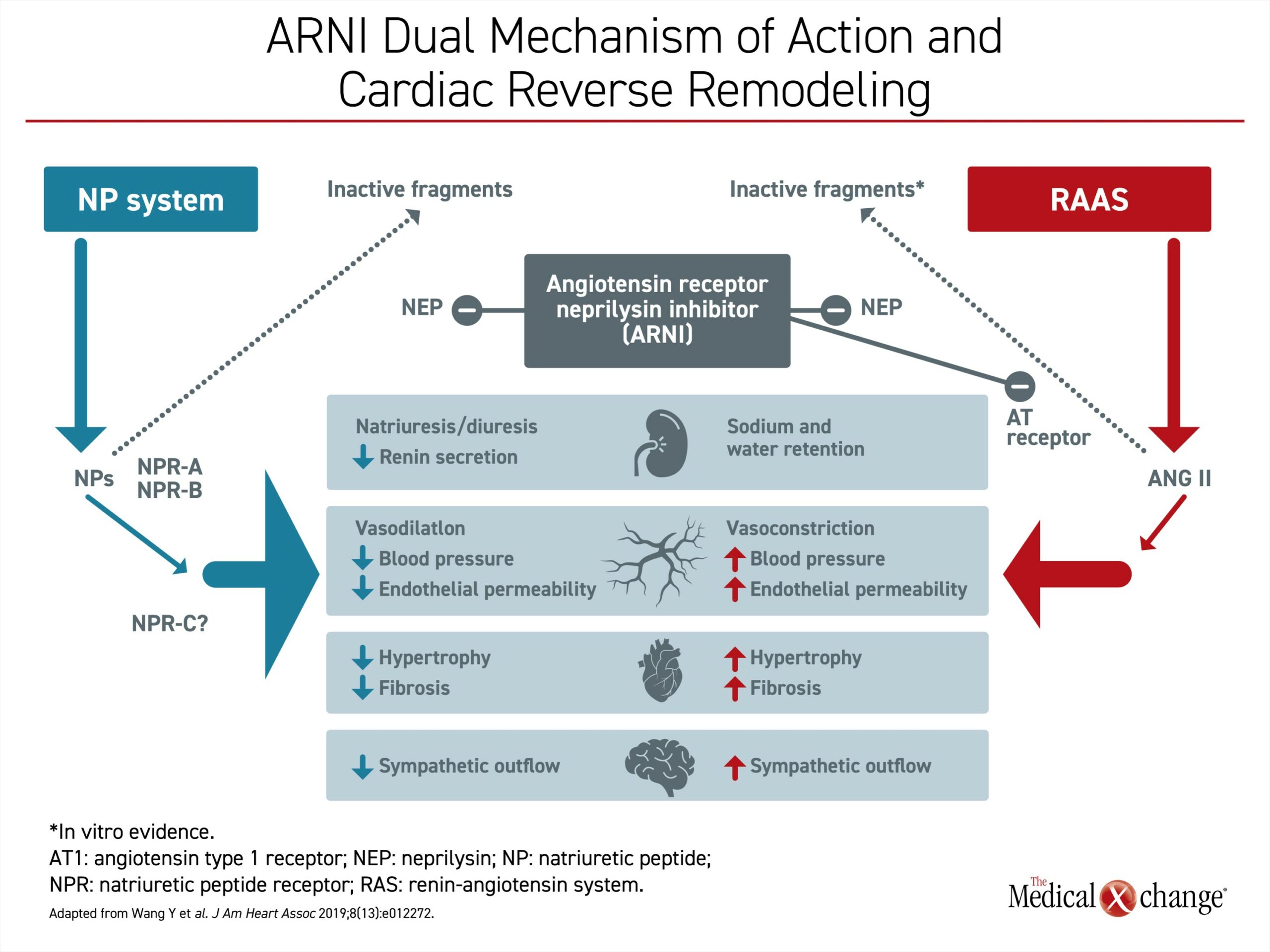
These benefits make mechanistic sense. Each GDMT modulates a different pathophysiologic mechanism of heart failure progression, providing an additive benefit. In the case of BB, the primary target is the sympathetic nervous system.18 MRA inhibits aldosterone, which is involved in the pathogenesis of cardiovascular fibrosis.19 The target for RASi is inhibition of angiotensin, a mediator of vasoconstriction.20 Each, among other effects, blocks major pathways of adverse cardiac remodeling. The advantage of the ARNI over RASi is attributed to its dual mechanisms.21 In addition to the inhibition of angiotensin, the ARNI blocks neprilysin, an enzyme that degrades natriuretic peptides. Both actions inhibit the neurohormonal upregulation that participates in cardiac remodeling (Figure 2).21 SGLT2i also improve outcomes in patients with HF directly and indirectly through several pathways involving renal effects, vascular function, and mediators of cardiac stress.22
CAN-HF Registry: Preventable Deaths Attributable to Suboptimal Implementation of GDMT
Previous studies, including those conducted in Canada, have shown that GDMT despite well-defined survival benefits are not being used consistently in HFrEF. In the US CHAMP-HF registry which included three and a half thousand patients with HFrEF, numerous patients were not prescribed ARNIs, beta blockers and MRAs (86%, 33% and 67%, respectively).23 In Canada, a national survey showed that one year after Canadian heart failure guidelines were modified to include an ARNI as a foundational GDMT, more than 85% of eligible patients were not receiving the treatment.24 The authors calculated that this omission was not only leading to preventable deaths but an estimated $40 million in annual costs related to avoidable heart failure hospitalizations.
According to more recent studies of heart failure GDMT in clinical practice, the problem persists.1 For a disease that is the single most important cause of cardiovascular death in Canada and where more than 100,000 new cases are diagnosed annually,25 a published study based on the CAN-HF registry reinforced other evidence that the majority of HFrEF patients are eligible for all four GDMT but a substantial proportion of those eligible do not receive one or more therapies.1
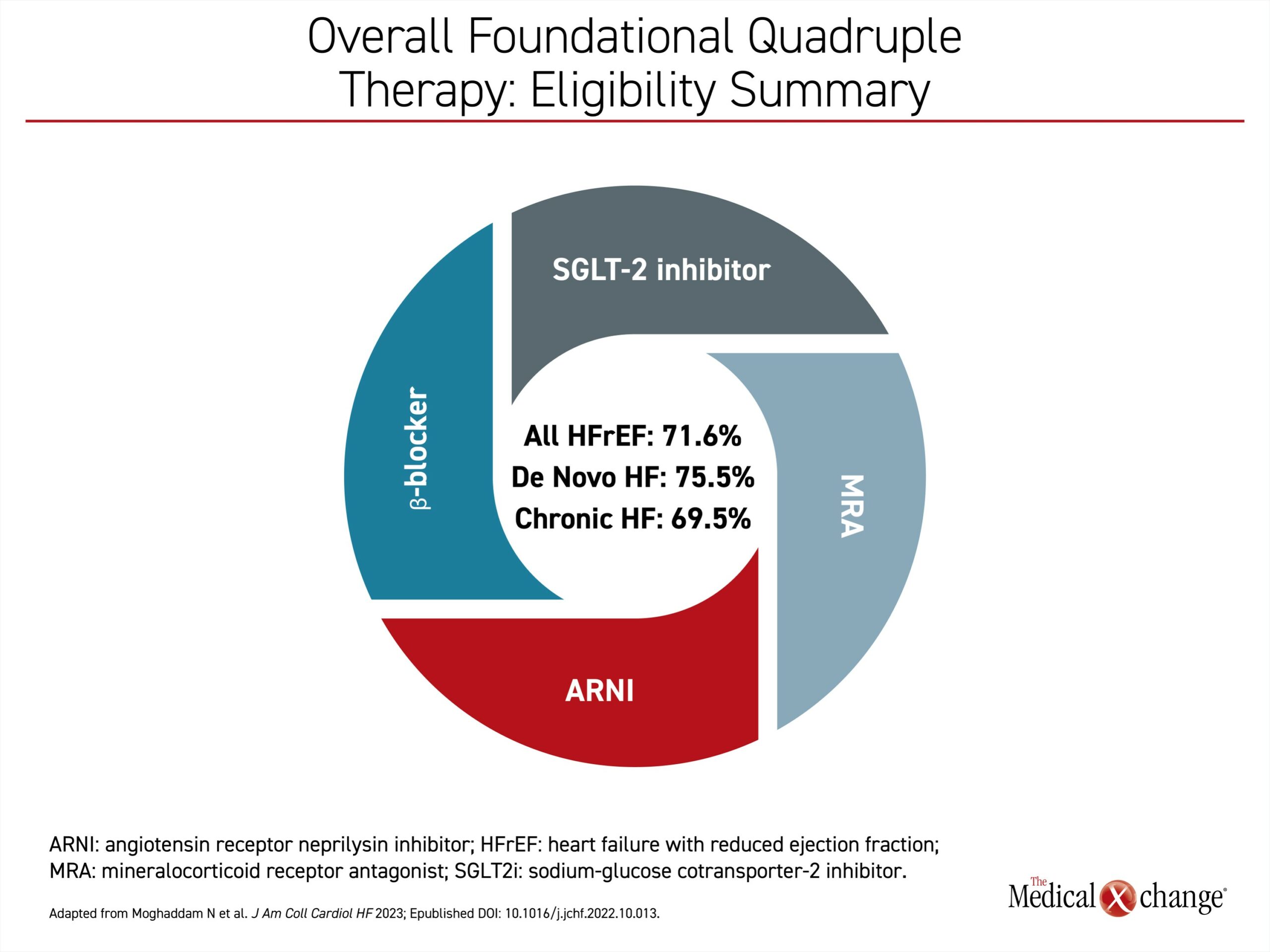
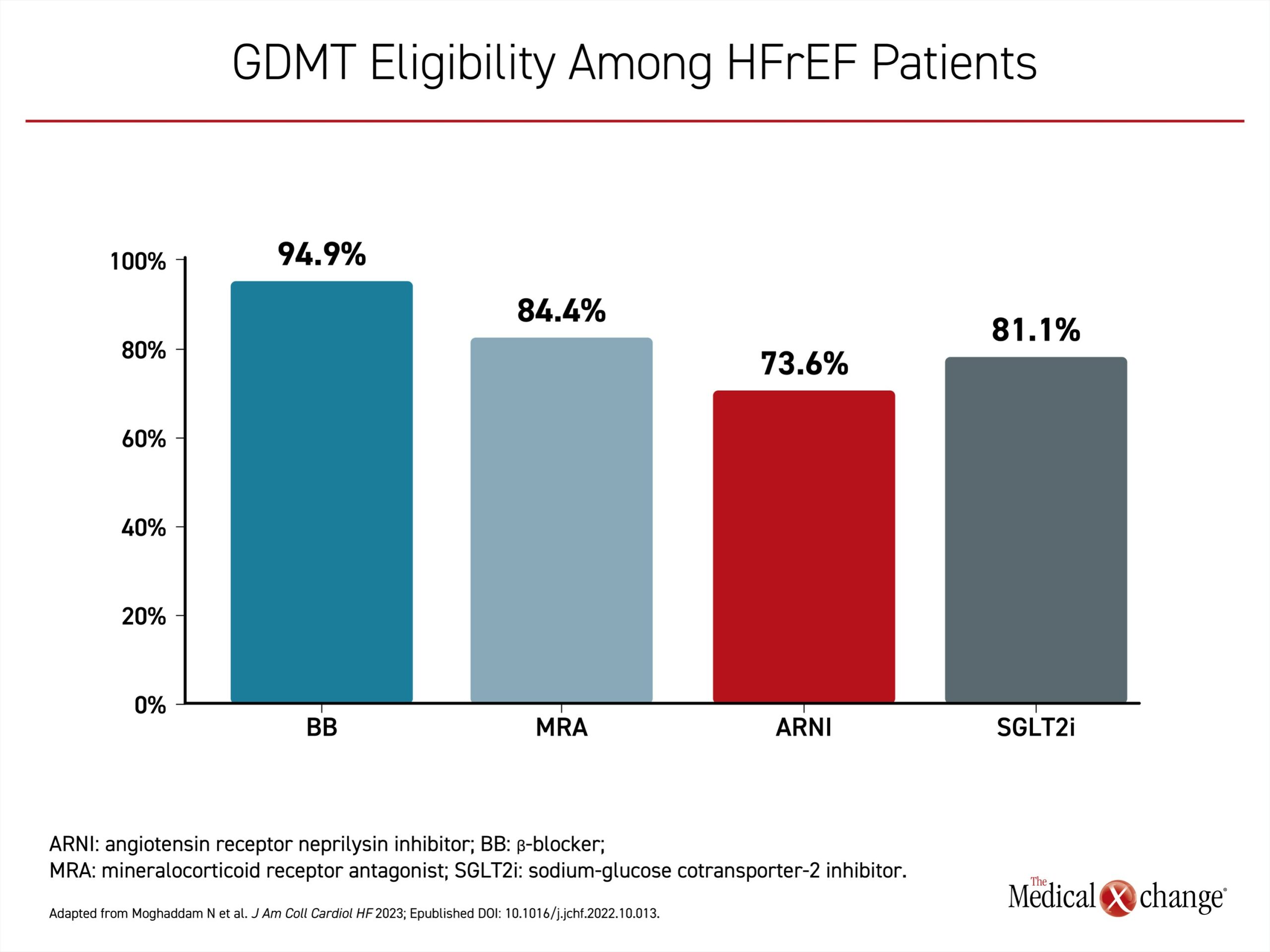
In this study, 809 newly diagnosed patients with heart failure treated at seven Canadian sites over a 3-year period (2017-2020) were evaluated.1 Of these, 455 (56%) had HFrEF, defined as left ventricular ejection fraction (LVEF) ≤40% and the remainder had LVEF >40%. Of the HFrEF patients, the vast majority, whether newly diagnosed (de novo) or with a prior history of HFrEF (chronic), were found to be eligible for the foundational therapies. In total, 75.5% of all de novo patients and 69.5% of chronic HFrEF patients were eligible for all four GDMTs (Figure 3). In patients hospitalized with HFrEF, the rates of eligibility for initiating specific types of GDMT were 73.6% for ARNI, 94.9% for beta blockers, 84.4% for MRA, and 81.1% for SGLT2i (Figure 4).
Among patients in the CAN-HF registry considered ineligible for one or more of the four GDMTs, the most common reasons were hypotension and renal dysfunction. All of the foundational GDMTs lower blood pressure to varying degrees, so this concern might be justified in some patients, but the risk of hypotension is likely insufficient in most instances to deny the potential survival benefits on optimized GDMT. Similarly, the potential for therapies to exacerbate declining rates of creatinine clearance cannot be ignored, however, screening patients for risk of hypotension and renal insufficiency should permit a treatment plan that allows for use of all the GDMT agents in most patients. This suggests that withholding potentially life-saving therapies can often be avoided.
Overall, the data indicate that clinicians may not be adhering to expert consensus that recommends initiating all components of GDMT prior to discharge except in selected individuals with established contraindications. This is inconsistent with the 2021 Canadian guidelines which state that in the absence of contraindications, initiation of one evidence-based therapy from each of the GDMT classes is recommended as soon as feasible after the diagnosis.2
Guideline Changes Emphasize Prompt Initiation
In the 2021 Canadian guidelines, several strategies were outlined to aid clinicians in prompt initiation of GDMT. These were included intentionally to place greater emphasis on eliminating delays.2 Up until 2017, a stepwise approach to the introduction of GDMT, starting with RASi and proceeding more or less in the order that the foundational therapies were established as part of GDMT was common. In this stepwise approach, adding and titrating treatments slowly, the delay to optimized therapy produced a substantial risk of events.
In 2017 when the ARNI was added to GDMT, the potential for delay was further exacerbated by the need for a washout in patients already taking an ACEi. Due to the risk of angioedema associated with an abrupt transition from ACEi to ARNI, a delay of 36 hours from the time of ACEi discontinuation until the initiation of an ARNI was recommended. This delay is not needed for patients previously taking an ARB, but the extra step from ACEi to ARNI was widely considered to prevent prompt ARNI initiation or, even worse, to the omission of the ARNI following the diagnosis of HFrEF.1,23,24 The newest Canadian guidelines address the importance of rapid initiation and uptitration of all four components of GDMT.2
In the guidelines, the PIONEER-HF trial cited in support of the value of rapid initiation of ARNI,12 which is now being described as a first-line therapy that is appropriate for in-hospital initiation.2 In this randomized trial, initiation of ARNI at the time of hospitalization for HFrEF was associated with better outcomes, including fewer rehospitalizations for heart failure, when employed with GDMT relative to the ACEi enalapril plus GDMT, which provided the control arm. The immediacy of neurohormonal modulation with prompt initiation of ARNI was reflected by the rapid reduction in elevated levels of NT-proBNP, a biomarker of neurohormonal activation, hemodynamic stress, and subsequent cardiovascular events.
The same point was made in a 2021 American College of Cardiology (ACC) update to a consensus decision pathway for HFrEF originally published jointly in 2017 by the ACC and the American Heart Association (AHA).26 The 2017 ACC/AHA guidelines included the same GDMT recommendations as that outlined in the 2017 Canadian guidelines,2,27 but the 2021 update, like the 2021 Canadian guidelines revision, addressed common delays in promptly initiating and uptitrating GDMT.
Also parallel to the 2021 Canadian guidelines, the 2021 ACC update characterized immediate initiation of the ARNI as safe and effective among patients who have not yet started RASi.26 For those already on an ACEi, the same 36-hour washout was recommended, but both guidelines state that this step should not be a rationale for a delay in initiating ARNI.
Aggregate Benefit of GDMT
Although ARNI has been shown repeatedly to be the most commonly omitted GDMT from current practice, the same urgency applies to the other agents, including SGLT2i, the most recent class to be added to GDMT for HFrEF. In the double-blind EMPULSE trial, patients hospitalized for acute heart failure were randomized to empagliflozin or placebo.28 Using hierarchical primary endpoint of all-cause death, a heart failure event, and time to a heart failure event, the win ratio of 1.36 for prompt initiation of SGLT2i over placebo was highly significant (P=0.0054). The study was not limited to HFrEF, and the benefit was observed regardless of ejection fraction.
In another trial called SOLOIST-WHF, more than 1200 patients with diabetes mellitus and heart failure were randomized to sotagliflozin (a combined SGLT1/SGLT2 inhibitor) or placebo administered prior to discharge (48.8%), or a median of 2 days post-discharge.29 Although the study was terminated early due to loss of funding, early initiation of sotagliflozin relative to placebo was associated with a significantly lower total number of deaths from cardiovascular causes and urgent visits for heart failure, which was the primary outcome.
The emphasis on rapidly initiating and uptitrating GDMT in HFrEF is based on compelling evidence of a cumulative benefit. In a meta-analysis of 75 trials with almost 100,000 patients, the combination of ACEi, BB, MRA, ARNI, and SGLT2i was associated with a more than 60% reduction in all-cause mortality with tight confidence intervals (HR 0.39, 95% CI 0.31 – 0.49) relative to no therapy.17 This combination was associated with similar reductions in risk for cardiovascular death and hospitalization for HF.
In a comparative analysis to evaluate the impact of more, relative to fewer GDMT agents, the reduction in all‑cause mortality was nearly 50% for the three-drug relative to the two-drug combination (HR 0.53, 95% CI 0.40 – 0.70).16 Comparable reductions were estimated for heart failure hospitalization, and several composite endpoints, such as cardiovascular death and hospitalization for heart failure. Based on these data, it was estimated that the four-drug combination extends life by an average of 6.3 additional years in a 55-year-old patient with HFrEF.
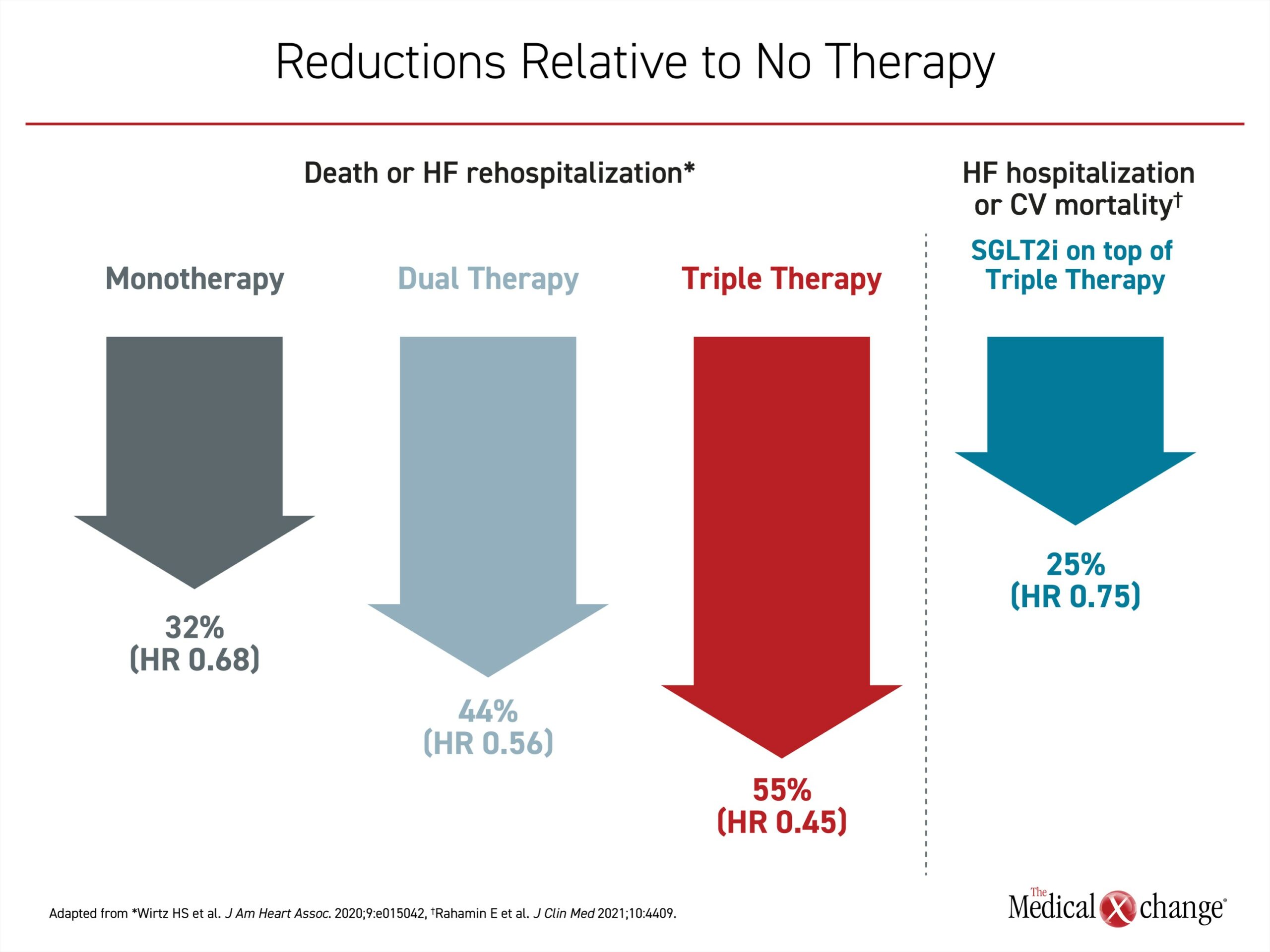
In a real-world retrospective observational analysis of GDMT after hospitalization for heart failure, the risk of the composite primary outcome of death or rehospitalization for heart failure following a four-drug GDMT with BB, MRA, and ARNI or RASI was reduced by 55% relative to no therapy (HR 0.45, 95% CI 0.41 – 0.50).30 The study showed a stepwise reduction in this endpoint with monotherapy (32%), dual therapy (44%), and triple therapy (55%) (Figure 5).
In the CAN-HF analysis, the authors also looked at potential missed benefits in patients with LVEF >40%.1 The benefits of these medications are not as well established in patients with heart failure with preserved ejection fraction (HFpEF), but they estimated that up to half of such patients are eligible for ARNI on the basis of the PARAGON-HF trial and up to 80% are eligible for MRA based on the TOPCAT trial.31,32 The authors cautioned that further analysis is needed to optimize patient selection for these therapies but stated that evidence-based medicine has the potential to further expand opportunities to improve outcomes in patients hospitalized with acute heart failure.
Together, the evidence cited in recent documents, including the newly published analysis, the 2021 Canadian guidelines, and the 2021 ACC consensus decision pathway update support an intensive approach to the management of HFrEF.2,26 Rather than a conservative and stepwise approach, all four GDMTs should be started as early as feasible, including during the index hospitalization. Following initiation, efforts should be timely to reach target doses. Overall, GDMT therapies are well tolerated and safe when conventional dosing schedules are followed. For eligible patients, the optimal survival benefit relies on all foundational GDMT.
Summary
Each of the four foundational therapies for HFrEF have survival benefits independent of the other three and have been well described in guidelines. However, delays in initiating treatment and reaching target doses have been reported in the recently published CAN-HF registry analysis and are a source of preventable deaths. The delays in replacing RASi with an ARNI is a frequent source of inadequate HFrEF treatment, but other GDMT therapies are also not promptly optimized in HFrEF, such as delays in initiating SGLT2i. The risk is preventable death.
References
- Moghaddam N, Hawkins NM, McKelvie R, Poon S. Patient eligibility for established and novel guideline-directed medical therapies after acute heart failure hospitalization. JACC HF 2023;11;S2213-1779(22)00638-2.DOI: 10.1016/j.jchf.2022.10.013.
- McDonald M, Virani S, Chan M, et al. CCS/CHFS Heart Failure Guidelines Update: Defining a New Pharmacologic Standard of Care for Heart Failure With Reduced Ejection Fraction. Can J Cardiol 2021;37(4):531-546. DOI: 10.1016/j.cjca.2021.01.017.
- Investigators S, Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991;325(5):293-302. DOI: 10.1056/NEJM199108013250501.
- Pfeffer MA, Braunwald E, Moye LA, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and
ventricular enlargement trial. The SAVE Investigators. N Engl J Med 1992;327(10):669-77. DOI: 10.1056/NEJM199209033271001. - Lechat P, Packer M, Chalon S, Cucherat M, Arab T, Boissel JP. Clinical effects of beta-adrenergic blockade in chronic heart failure: a metaanalysis of double-blind, placebo-controlled, randomized trials. Circulation 1998;98(12):1184-91. DOI: 10.1161/01.cir.98.12.1184.
- Zannad F, Gattis Stough W, Rossignol P, et al. Mineralocorticoid receptor antagonists for heart failure with reduced ejection fraction: integrating evidence into clinical practice. Eur Heart J
2012;33(22):2782-95. DOI: 10.1093/eurheartj/ehs257. - Peterson RC, Dunlap ME. Angiotensin II receptor blockers in the treatment of heart failure. Congest Heart Fail 2002;8(5):246-50; 256. DOI: 10.1111/j.1527-5299.2000.01156.x.
- Ezekowitz JA, O’Meara E, McDonald MA, et al. 2017 Comprehensive Update of the Canadian Cardiovascular Society Guidelines for the Management of Heart Failure. Can J Cardiol 2017;33(11):1342-1433. DOI: 10.1016/j.cjca.2017.08.022.
- McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371(11):993-1004. DOI: 10.1056/NEJMoa1409077.
- Pascual-Figal D, Bayes-Genis A, Beltran-Troncoso P, et al. Sacubitril-Valsartan, Clinical Benefits and Related Mechanisms of Action in Heart Failure With Reduced Ejection Fraction. A Review. Front Cardiovasc Med 2021;8:754499. DOI: 10.3389/fcvm.2021.754499.
- McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med 2019;381(21):1995-2008. DOI: 10.1056/NEJMoa1911303.
- Anker SD, Butler J, Filippatos G, et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N Engl J Med 2021;385(16):1451-1461. DOI: 10.1056/NEJMoa2107038.
- Keller DM, Ahmed N, Tariq H, et al. SGLT2 Inhibitors in Type 2 Diabetes Mellitus and Heart Failure-A Concise Review. J Clin Med 2022;11(6). DOI: 10.3390/jcm11061470.
- Vaduganathan M, Docherty KF, Claggett BL, et al. SGLT-2 inhibitors in patients with heart failure: a comprehensive meta-analysis of five randomised controlled trials. Lancet 2022;400(10354):757-767. DOI: 10.1016/S0140-6736(22)01429-5.
- Rahamim E, Nachman D, Yagel O, et al. Contemporary Pillars of Heart Failure with Reduced Ejection Fraction Medical Therapy. J Clin Med 2021;10(19). DOI: 10.3390/jcm10194409.
- Vaduganathan M, Claggett BL, Jhund PS, et al. Estimating lifetime benefits of comprehensive disease-modifying pharmacological therapies in patients with heart failure with reduced ejection fraction: a comparative analysis of three randomised controlled trials. Lancet 2020;396(10244):121-128. DOI: 10.1016/S0140-6736(20)30748-0.
- Tromp J, Ouwerkerk W, van Veldhuisen DJ, et al. A Systematic Review and Network Meta-Analysis of Pharmacological Treatment of Heart Failure With Reduced Ejection Fraction. JACC Heart Fail 2022;10(2):73-84. DOI: 10.1016/j.jchf.2021.09.004.
- Barrese V, Taglialatela M. New advances in beta-blocker therapy in heart failure. Front Physiol 2013;4:323. DOI: 10.3389/ fphys.2013.00323.
- Leopold JA. Aldosterone, mineralocorticoid receptor activation, and cardiovascular remodeling. Circulation 2011;124(18):e466-8. DOI: 10.1161/CIRCULATIONAHA.111.067918.
- Ferrario CM. Cardiac remodelling and RAS inhibition. Ther Adv Cardiovasc Dis 2016;10(3):162-71. DOI: 10.1177/1753944716642677.
- Wang Y, Zhou R, Lu C, Chen Q, Xu T, Li D. Effects of the Angiotensin- Receptor Neprilysin Inhibitor on Cardiac Reverse Remodeling: Meta-Analysis. J Am Heart Assoc 2019;8(13):e012272. DOI: 10.1161/ JAHA.119.012272.
- Lopaschuk GD, Verma S. Mechanisms of Cardiovascular Benefits of Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitors: A State-of-the- Art Review. JACC Basic Transl Sci 2020;5(6):632-644. DOI: 10.1016/j. jacbts.2020.02.004.
- Greene SJ, Butler J, Albert NM, et al. Medical Therapy for Heart Failure With Reduced Ejection Fraction: The CHAMP-HF Registry. J Am Coll Cardiol 2018;72(4):351-366. DOI: 10.1016/j.jacc.2018.04.070.
- Huitema AA, Daoust A, Anderson K, et al. Optimal Usage of Sacubitril/Valsartan for the Treatment of Heart Failure: The Importance of Optimizing Heart Failure Care in Canada. CJC Open 2020;2(5):321-327. DOI: 10.1016/j.cjco.2020.03.015.
- Poon S, Leis B, Lambert L, et al. The State of Heart Failure Care in Canada: Minimal Improvement in Readmissions Over Time Despite an Increased Number of Evidence-Based Therapies. CJC Open 2022;4(8):667-675. DOI: 10.1016/j.cjco.2022.04.011.
- Maddox TM, Januzzi JL, Jr., Allen LA, et al. 2021 Update to the 2017 ACC Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment: Answers to 10 Pivotal Issues About Heart Failure With Reduced Ejection Fraction: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol 2021;77(6):772-810. DOI: 10.1016/j.jacc.2020.11.022.
- Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/ American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017;136(6):e137-e161. DOI: 10.1161/CIR.0000000000000509.
- Kosiborod MN, Angermann CE, Collins SP, et al. Effects of Empagliflozin on Symptoms, Physical Limitations, and Quality of Life in Patients Hospitalized for Acute Heart Failure: Results From the EMPULSE Trial. Circulation 2022;146(4):279-288. DOI: 10.1161/ CIRCULATIONAHA.122.059725.
- Bhatt DL, Szarek M, Steg PG, et al. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. N Engl J Med 2021;384(2):117-128. DOI: 10.1056/NEJMoa2030183.
- Wirtz HS, Sheer R, Honarpour N, et al. Real-World Analysis of Guideline-Based Therapy After Hospitalization for Heart Failure. J Am Heart Assoc 2020;9(16):e015042. DOI: 10.1161/JAHA.119.015042.
- Velazquez EJ, Morrow DA, DeVore AD, et al. Angiotensin-Neprilysin Inhibition in Acute Decompensated Heart Failure. N Engl J Med 2019;380(6):539-548. DOI: 10.1056/NEJMoa1812851.
- Cikes M, Claggett B, Shah AM, et al. Atrial Fibrillation in Heart Failure With Preserved Ejection Fraction: The TOPCAT Trial. JACC Heart Fail 2018;6(8):689-697. DOI: 10.1016/j.jchf.2018.05.005.