Hematology
American Society of Clinical Oncology (ASCO) 2023 Annual Meeting
BTK Inhibitors Differentiated on Safety and Efficacy in CLL
Chicago – Retrospective analyses presented at the annual ASCO meeting attempted to demonstrate the viability of a first-generation Bruton tyrosine kinase inhibitor (BTKi) despite off-target effects and the need for dose adjustments to manage cardiac events. Head-to-head trials such as ALPINE and ELEVATE R/R have demonstrated that second-generation BTKi agents are better tolerated and in the case of the ALPINE trial, demonstrated superior clinical efficacy. By placing these retrospective analyses within the context of these major trials, a more comprehensive understanding of treatment choices and their implications are gained.
The first-generation BTKi ibrutinib was a revolutionary drug for chronic lymphocytic leukemia (CLL) / small lymphocytic lymphoma (SLL) and many other B-cell malignancies when it became available more than a decade ago, but there is now overwhelming evidence that BTK specificity, which distinguishes second-generation agents, matters. The retrospective analyses were based on electronic medical record (EMR) reviews or pooled trial analyses. Although the analyses attempt to explain why ibrutinib remains a viable choice despite off-target effects, there are now prospective, multicenter, well controlled trials that provide compelling evidence for the superiority of second-generation agents.
First-generation BTK Inhibitor Associated with Dose Adjustments to Manage Adverse Events
Two retrospective studies presented at ASCO examined the use of ibrutinib dose adjustments to reduce the impact of cardiotoxicity or other off-target effects.
In one study, EMRs of 1,171 CLL/SLL patients were drawn from the Acentrus database to evaluate the impact of dose adjustments on time to next treatment (TTNT). Attributed to off-target effects, dose adjustments were required in 19.6% of the population overall and in 21.3% of those considered to be at high risk of cardiovascular (CV) events. Half of these dose adjustments occurred within the first 6 months.
At 12 months of follow-up, only a small proportion of patients overall (10.9% vs. 7.5%; P=0.254) or at high CV risk (7.3% vs. 8.0%; P=0.774) had advanced to another treatment, reported Dr. Kerry A. Rogers, Assistant Professor, Division of Hematology, Ohio State University, Columbus. While she concluded that this is a “real-world demonstration of [ibrutinib’s] dosing flexibility,” it fails to acknowledge that second-generation BTKi agents have a lower risk of toxicities requiring dosing adjustments.
Impact of Dose Reductions on Progression-free Survival (PFS) Outcomes
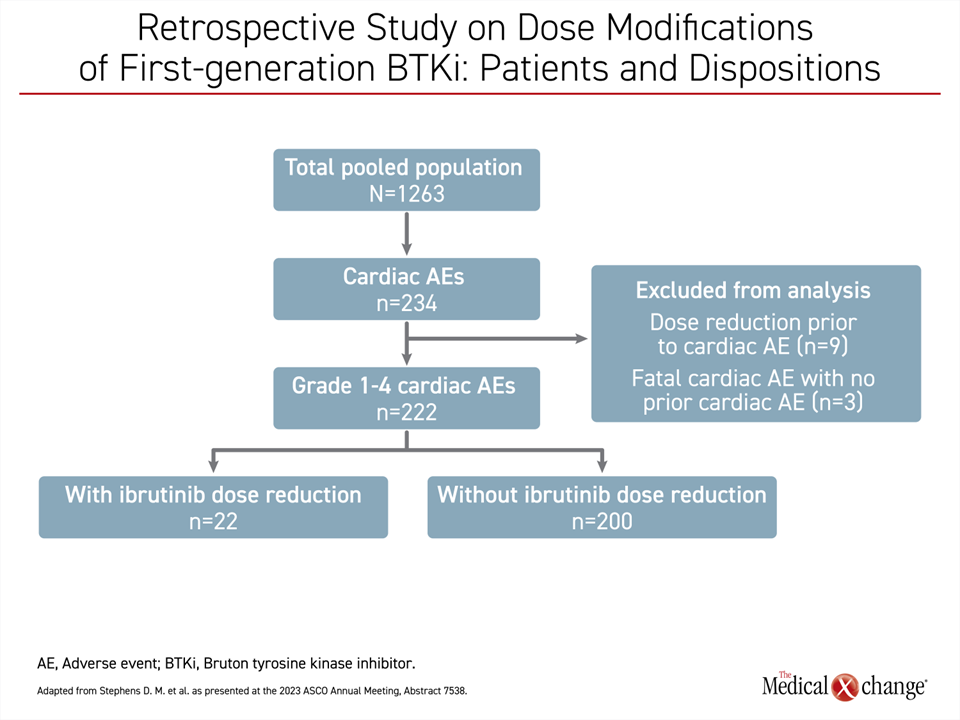
Similar support for ibrutinib was based on another retrospective study. In this study, 1,263 patients were pooled from 10 clinical trials with ibrutinib in CLL/SLL or another B-cell malignancy. The objective was to evaluate the impact on PFS produced by dose adjustments for the management of cardiac events, reported Dr. Deborah M. Stephens, Assistant Professor, Division of Hematology and Hematologic Malignancies, University of Utah, Salt Lake City.
In this study population, 222 patients (17.6%) had an adverse cardiac event of which 22 (9.9%) had ibrutinib dose reduction (Figure 1). While the analysis excluded patients with dose adjustments for another reason and the 3 patients who had a fatal cardiac event prior to a dose adjustment, the study found a slightly higher rate of PFS at 24 months for those with a dose reduction for adverse events per prescribing information recommendations than without (86% vs. 76%) even though the difference was not statistically significant.
The take-home message, according to Dr. Stephens, is the PFS is “not negatively impacted” by dose adjustments for cardiotoxicity. However, the studies compared the impact of dose adjustments only among patients taking ibrutinib. As such, it is important to approach the interpretation of this analysis with caution since it lacks a comparison to a second-generation BTK inhibitor, known for its safety advantage.
Phase 3 Trial Confirms Superior PFS Benefit of Second-generation BTK Inhibitor
ALPINE and ELEVATE R/R were head-to-head comparisons of a second-generation BTKi to ibrutinib for patients with previously treated CLL. While both trials confirmed a safety advantage relative to the less specific ibrutinib, the data presented differences.
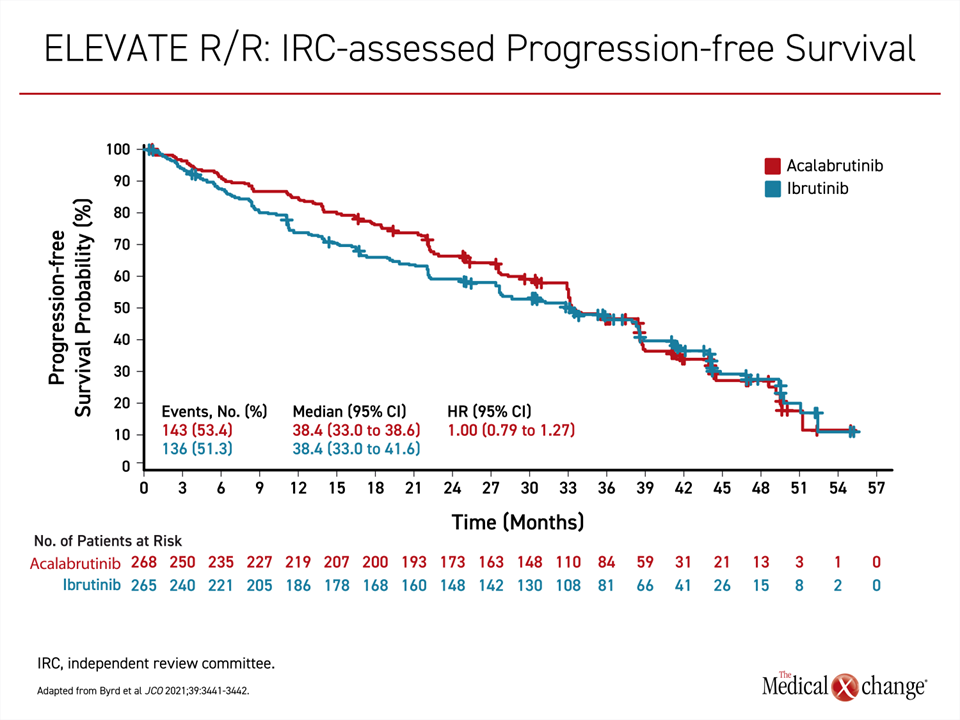
In the randomized open-label ELEVATE R/R, published in 2021, acalabrutinib was non-inferior to ibrutinib for the primary endpoint of PFS (Byrd et al. J Clin Oncol 2021;39:3441-3452). In fact, the median PFS after more than 3 years of follow-up was an identical 38.4 months in both arms (P=0.002) with a hazard ratio of 1.00 (Figure 2).
Yet, the risk of CV adverse events, particularly atrial fibrillation and flutter was lower in the acalabrutinib arm compared to the ibrutinib arm (9.4% vs. 16.0%; P=0.02), and was regarded as clinically meaningful as well as proof of the advantage of second-generation BTKi. The greater BTK specificity is the hypothesized explanation for the favourable safety profile.
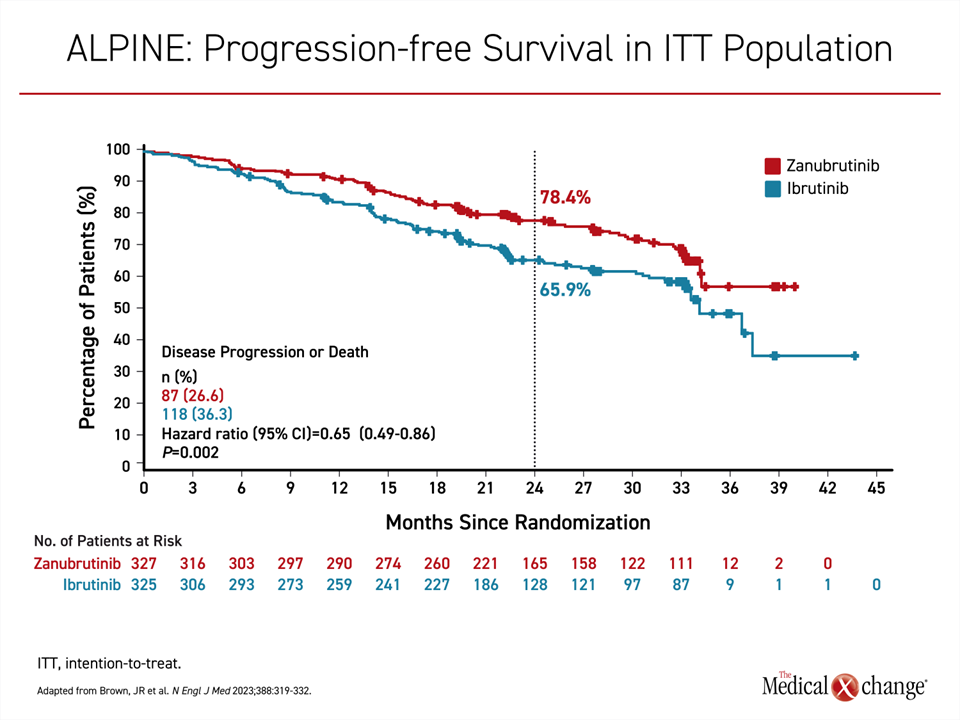
In the more recent randomized and open-label ALPINE trial, published earlier this year and presented at the American Society of Hematology (ASH) 2022 meeting, zanubrutinib was superior to ibrutinib for the endpoint of PFS (Brown et al. N Engl J Med 2023;388:319-332). At a median follow-up of 29.6 months, the median PFS had not been reached in the zanubrutinib arm versus 34.2 months in the ibrutinib arm. The relative reduction in favour of zanubrutinib for progression or death was 35% (HR 0.65; P=0.002) (Figure 3).
As observed in the phase 3 trial with acalabrutinib, zanubrutinib was associated with a lower incidence of CV disorders overall (21.3% vs. 29.6%) as well as atrial fibrillation specifically (1.9% vs. 3.7%). Grade 3 or higher adverse events, neutropenia (16.0% vs. 13.9%) and hypertension (14.8% vs. 11.1%) were slightly more common on zanubrutinib, but adverse events leading to a dose reduction (12.3% vs. 17.0%) or treatment discontinuation (15.4% vs. 22.2%) were less frequent. Six deaths due to cardiac events were reported, all in patients who received ibrutinib.
ALPINE: Favourable Responses in High-risk Genetic Subgroups
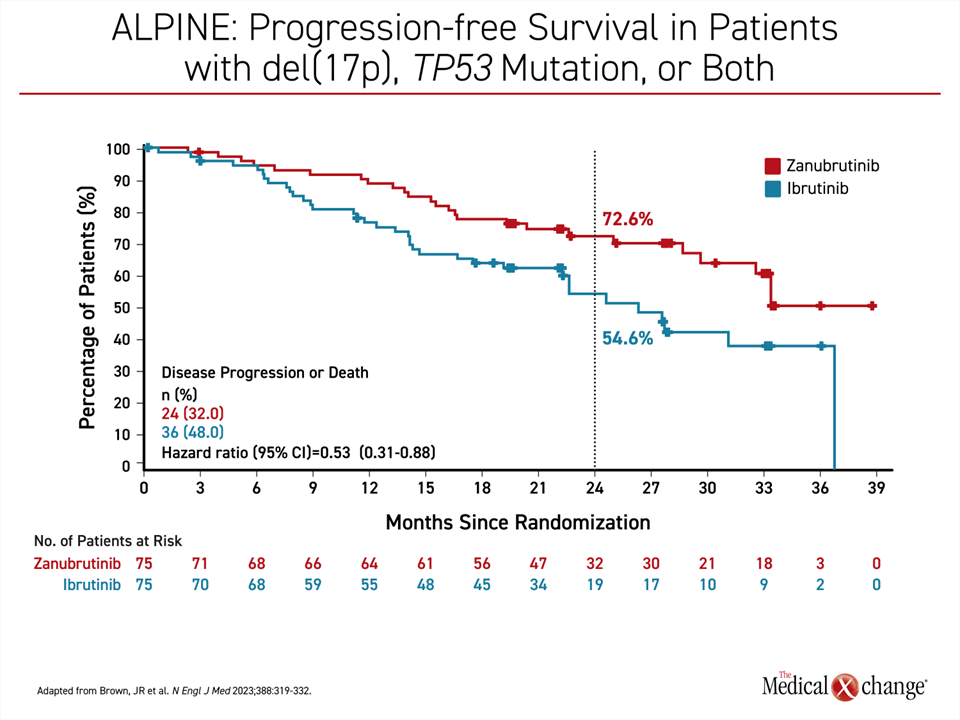
The study populations in ELEVATE R/R and ALPINE were not identical. In particular, a del(17)p13.1 and/or a del(11)q22.3 mutation was an enrollment requirement in ELEVATE R/R. In ALPINE, CLL patients were eligible regardless of genomic findings. When the subgroup of ALPINE patients with high-risk CLL, defined as disease with a 17p deletion, TP53 mutation, or both, was analyzed separately, at 24 months, the percentage of patients with PFS was about the same as that observed in the intention-to-treat population (72.6% vs. 78.4%). The efficacy of ibrutinib in the high-risk subgroup of ALPINE was substantially reduced compared to the intention-to-treat population (54.6% vs. 65.9%) (Figure 4).
As a result, reduction for progression or death was even greater with zanubrutinib relative to ibrutinib in the high-risk group, approaching 50% (HR 0.53; (95% CI, 0.31 – 0.88). At 24 months, 72.6% of zanubrutinib-treated patients were alive and remained progression free versus 54.6% of those randomized to ibrutinib. A numerical OS benefit was reported at early follow-up but did not reach statistical significance, a longer follow-up is needed (HR 0.76; (95% CI, 0.51-1.11). In ELEVATE R/R, median OS was not reached in either arm (HR 0.82, 95% CI 0.59 – 1.15), a separation in the OS curve at about 27 months also remained insignificant.
Benefits of Once-daily Dosing
In the ALPINE and ELEVATE R/R trials, zanubrutinib and acalabrutinib were prescribed in twice-daily doses of 160 mg and 100 mg, respectively. However, once-daily dosing of 320 mg is an option under labeling granted by the U.S. Food and Drug Administration and Health Canada. This is based on complete BTK occupancy in peripheral blood cells and pharmacokinetics, according to Dr. Jennifer R. Brown, Director of the CLL Center, Dana-Farber Cancer Institute, Boston, Massachusetts, who described studies that demonstrated these properties. The support for a once-daily 320-mg dose outside of the ALPINE trial might be relevant to selected individuals, such as those with reduced compliance to a twice-daily drug.
In fact, better compliance was offered as an explanation for the relative persistence of benefit among ibrutinib patients who underwent ibrutinib dose reductions in the retrospective analysis of EMR data presented by Dr. Rogers. In her descriptive analysis, Dr. Rogers noted that patients whose dose was reduced as a result of toxicities “had higher adherence […] relative to those who remained on the 420 mg/day starting dose”. This might account for the comparable TTNT results.
On the basis of separate trials, no conclusions about the relative safety and efficacy of the two second-generation BTKi is appropriate, but there is now compelling multicenter evidence that the BTK specificity of zanubrutinib and acalabrutinib is clinically meaningful. Both agents have shown a lower risk of cardiotoxicity, which is arguably the most meaningful adverse event associated with the first-generation BTKi. In addition, zanubrutinib demonstrated a substantial efficacy advantage over ibrutinib, further supporting the concept this second-generation BTKi supplants the first-generation drug.
Conclusion
Guidelines for the use of BTKi in CLL have been revised on the basis of two major trials that have shown that second-generation drugs with greater BTK specificity should be preferred over the first-generation agent. At this year’s ASCO meeting, several studies evaluated whether ibrutinib remains viable on the basis of retrospective data. However, the question of whether BTK specificity matters has already been answered by large well controlled multicenter trials designed to address this question. Despite the importance that ibrutinib has played in CLL, the second-generation agents zanubrutinib and acalabrutinib have convincingly shown greater safety in the management of CLL, while zanubrutinib has also shown greater efficacy.