Hematology
European Hematology Association (EHA)
2023 Hybrid Congress
Long-term Efficacy in CLL: Expanding Evidence Across Treatment-naïve and Relapsed/Refractory Patients
Frankfurt/Virtual – Presentations from the European Hematology Association (EHA) 2023 Congress shed light on the expanding evidence of differences between first- and second-generation Bruton tyrosine kinase inhibitors (BTKi) in the treatment of chronic lymphocytic leukemia (CLL). Notably, the long-term follow-up data from the SEQUOIA trial and results from the ALPINE health-related quality of life (HRQoL) assessment were presented and reaffirmed the benefit of the second-generation BTKi. Moreover, results highlighted the importance of BTK selectivity and occupancy in differentiating second-generation BTKi and their potential impact on safety and efficacy outcomes.
On the basis of two phase 3 multicenter trials, SEQUOIA and ALPINE, Health Canada recently extended the indication for zanubrutinib, a second-generation BTKi, to include treatment of both front-line and relapsed/refractory (R/R) CLL. Both trials showed an efficacy advantage and improved tolerability profile for this second-generation BTKi relative to a standard of care comparator.
For an exclusive interview with Dr. Talha Munir on the impact to clinical practice, click here
Front-line Efficacy of Second-generation BTK Inhibitor
When the SEQUOIA results were originally reported a year ago, zanubrutinib achieved a ≥50% reduction (HR 0.42; P<0.0001) in progression or death relative to the combination of bendamustine plus rituximab (BR) among patients with CLL or small lymphocytic lymphoma (SLL) (Tam CS et al. Lancet Oncology 2022;8:1031-1043). At the EHA congress, the 18 months of additional follow-up confirm the benefit persists over time with low relative rates of adverse events.
“The results of this extended follow-up support the use of zanubrutinib as a valuable first-line treatment option,” reported Dr. Talha Munir, Haematological Malignancy Diagnostic Service, St. James’s Institute of Oncology, Leeds, UK.
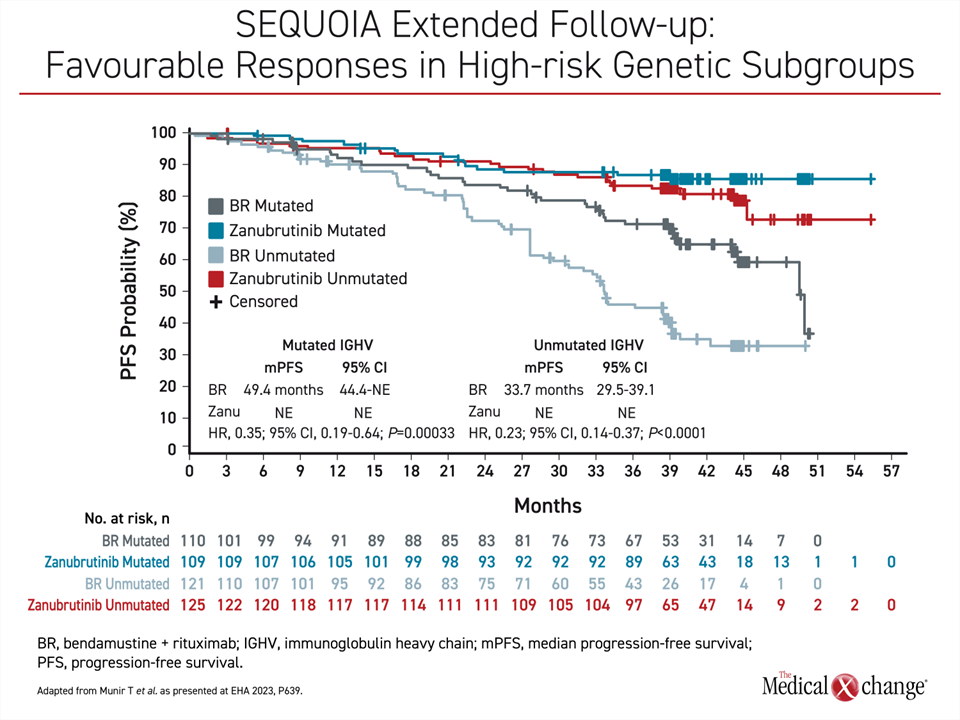
In SEQUOIA, 479 treatment-naïve patients without del(17p) were randomized to zanubrutinib or BR (cohort 1). An additional 111 patients with del(17p) were followed on zanubrutinib monotherapy (cohort 2). The median follow-up is nearly 4 years in both cohort 1 (43.7 months) and cohort 2 (47.9 months). In the randomized part of this trial, the median PFS was 42.2 months in the BR group but still has not been reached in the zanubrutinib group. This translates into a 70% relative reduction for the second-generation BTKi (HR 0.30; P<0.0001).
Furthermore, the advantage was similar for those with mutated IGHV or unmutated IGHV. While the median PFS has not been met in either zanubrutinib arm, the median PFS in the BR arm was 49.4 months in the mutated IGHV group and 33.7 months in the unmutated group. As a result, there is a relative reduction in the risk of death or progression with zanubrutinib compared to BR of 65% in the IGHV mutated patients (HR 0.35; P=0.00033) and 77% in the IGHV unmutated patients (HR 0.23; P<0.0001) (Figure 1).
“The results of this extended follow-up support the use of zanubrutinib as a valuable first-line treatment option.”
In cohort 2, the patients with a del(17p) mutation, 70.3% of zanubrutinib patients remain on therapy in follow-up to date. The median PFS on zanubrutinib has not been reached in this cohort, but the estimated rate at 42 months was 79.4%. This is only slightly lower than the 82.4% estimated PFS at 42 months for those without del(17p).
Continued Safety Advantage
As expected, there were modest differences in the rates and types of treatment-emergent adverse events (TEAE) for zanubrutinib and BR. Adverse events of interest for zanubrutinib were as expected for second-generation BTKi. Grade 3 or higher diarrhea (1.7% vs. 2.2%), neutropenia (12.5% vs. 51.1%), anemia (0.4% vs. 2.2) and thrombocytopenia (2.1% vs. 7.9%) were lower on zanubrutinib, but grade 3 or higher infections (23.8% vs. 22.0%), bleeding (5.8% vs. 1.8%) and hypertension (9.2% vs. 6.6%) were higher. Among those with del(17p), none of these side effects were substantially more common on zanubrutinib.
Of particular note in the SEQUOIA trial, which enrolled mostly older patients, atrial fibrillation (AF) and flutter, major hemorrhage, and hypertension remained low in patients randomized to BR or zanubrutinib. This is important because these adverse events are often a concern in patients on ibrutinib. The rates of grade 3 or higher AF/flutter in the zanubrutinib arms were 1.3% in patients without a del(17p) mutation and 4.5% in patients with a del(17p) mutation) and rates of grade 3 or higher hypertension were 6.6% and 9.2% respectively. Overall, Zanubrutinib was well tolerated over this extended treatment period and rates of atrial fibrillation events remained low.
Second-generation Agents Supersede First-generation BTK Inhibitor
The second-generation BTKi, which includes acalabrutinib and zanubrutinib, have proven to be safer relative to first-generation ibrutinib in trials, including phase 3 head-to-head comparisons. Notably, zanubrutinib also confirmed a superior efficacy compared to ibrutinib. Relative selectivity for the target kinase, more favourable pharmacokinetics, and increased BTK occupancy, are implicated in differentiating second- to first-generation agents and between second-generation agents.
In R/R CLL, ELEVATE R/R (Byrd J et al J Clin Oncol 2021;39:3441-3452) highlighted that BTK target selectivity is important by demonstrating a lower risk of cardiovascular (CV) events for the second-generation acalabrutinib relative to the first-generation ibrutinib. The median PFS was an identical 38.4 months in both arms (P=0.002) with a hazard ratio of 1.00.
The ALPINE trial was published earlier this year (Brown et al. N Engl J Med 2023;388:319-332). Like acalabrutinib in ELEVATE R/R, zanubrutinib was associated with a lower risk of CV events and other side effects hypothesized to be the result of off-target enzyme inhibition. In addition, zanubrutinib was shown to have superior PFS compared to ibrutinib. At 24 months, the PFS rates were 79.5% in the zanubrutinib group and 67.3% in the ibrutinib group. The relative reduction in favour of zanubrutinib for progression or death was 35% (HR 0.65; P=0.002).
“Zanubrutinib is a potent and selective next-generation BTKi that has been designed to improve tolerability by maximizing BTK occupancy and minimizing off-target effects,” reported Dr. Jennifer R. Brown, Department of Medical Oncology, Dana-Farber Cancer Institute, Boston. Dr. Brown was the principal investigator of the ALPINE trial, but she also led a safety analysis of zanubrutinib across multiple B-cell malignancies that was presented at the EHA.
“Zanubrutinib is a potent and selective next-generation BTKi that has been designed to improve tolerability by maximizing BTK occupancy and minimizing off-target effects.”
Safety Considerations: Selectivity of BTK Inhibitors in B-cell Malignancies
The safety data pooled from 10 studies, including the ALPINE trial, included 1,550 patients treated with zanubrutinib and 422 with ibrutinib. The majority of patients (60.5%) had CLL/SLL, but other diagnoses, including Waldenström’s macroglobulinemia, marginal cell lymphoma, follicular lymphoma, and diffuse large B-cell lymphoma were also included. About a third of patients treated with zanubrutinib were treatment-naïve, and almost 20% had received ≥3 prior lines of treatment.
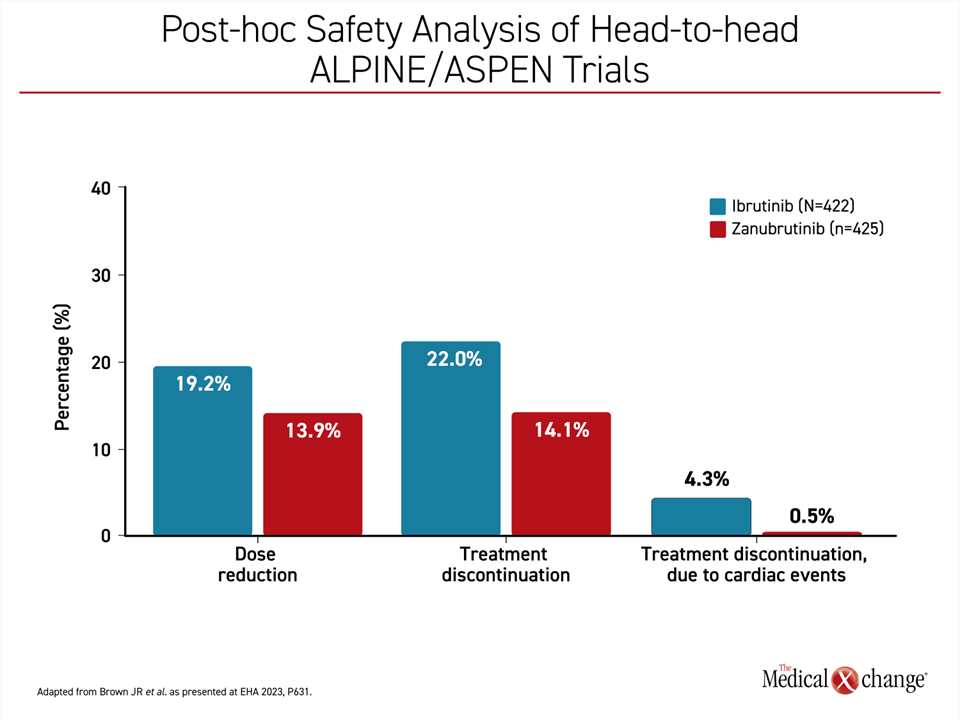
Non-cardiovascular adverse events among those treated with zanubrutinib relative to ibrutinib were similar when calculated as exposure-adjusted incidence rates, but hypertension and atrial fibrillation occurred more commonly on ibrutinib. The most common adverse events overall for zanubrutinib were upper respiratory tract infections, diarrhea, and contusion, but these were generally mild to moderate in severity. When confined to the two head-to-head comparisons of zanubrutinib and ibrutinib in this pooled analysis (ALPINE and ASPEN), dose reductions, treatment discontinuations, and treatment discontinuations due to a cardiac TEAE were all more common with ibrutinib (Figure 2). Cardiac-related TEAE leading to death were lower on zanubrutinib than with ibrutinib (0.2% vs. 1.7%) in the head-to-head populations.
“Due to the continuous dosing of BTKi in most B-cell malignancies, long-term tolerability and low treatment discontinuation rates with BTKi are important,” Dr. Brown pointed out, indicating that selectivity does not only provide a hypothesis for differences between second- and first-generation BTKi but is relevant to drugs in the second-generation class to the extent that it reduces off-target effects.
Quality of Life Considerations in Long-term Treatment of CLL
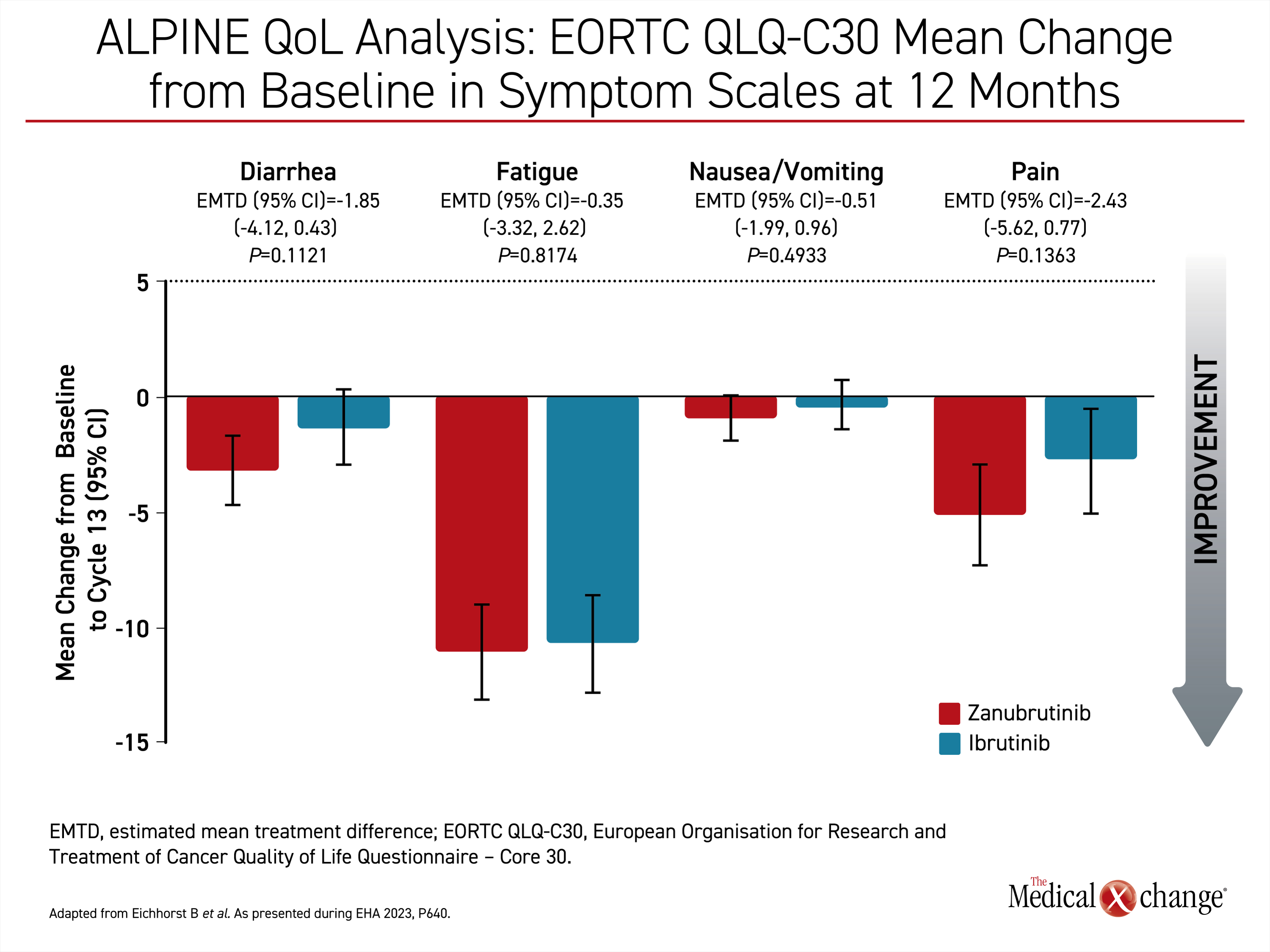
In other data relevant to disease control with second-generation BTKi, differences in HRQoL were observed between zanubrutinib and ibrutinib in the ALPINE trial. This secondary endpoint was based on the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire – Core 30 (EORTC QLQ-C30), which was administered at the ends of cycle 7 and 13.
“Both arms experienced a decrease in fatigue and pain with the zanubrutinib arm experiencing clinically meaningful improvements in both symptoms in both cycles,” reported Dr. Barbara Eichhorst, Associate Professor Division of Hematology, University of Cologne, Germany.
“Zanubrutinib [experienced] clinically meaningful improvements in both symptoms in both cycles.”
When compared for general health status, physical function, or role functioning, zanubrutinib was at least numerically superior to ibrutinib for all domains. The improvements were maintained from 6 months through 12 months, the cutoff point of these analyses (Figure 3).
Further studies to match QoL with efficacy, safety, and patient-reported outcomes (PRO) are planned, but Dr. Eichhorst indicated that improved QoL, which is dependent on both treatment control and relative freedom from treatment-related adverse events, is sometimes an overlooked part of the management of CLL and other B-cell malignancies. In early stages of disease, when QoL is generally maintained with few symptoms, a well-tolerated therapy sustains well-being, but BTKi are often taken indefinitely to control B-cell malignancies, emphasizing the value of minimizing adverse events, including off-target effects, that threaten QoL.
BTK Inhibitors Differentiated
The effort to differentiate second-generation agents for safety and efficacy has so far been indirect. In experimental studies, there are differences between these drugs. For example, kinase profiling demonstrates that “zanubrutinib has higher selectivity than ibrutinib, acalabrutinib, and acalabrutinib’s major metabolite,” according to Dr. Mazyar Shadman, Attending Physician, Fred Hutchinson Cancer Center, Seattle, Washington.
In addition, pharmacologic differences, such as the longer half-life of zanubrutinib has potential relevance to BTK occupancy. In data presented at the EHA, Dr. Shadman showed that a high proportion of patients intolerant to ibrutinib or acalabrutinib did not have a recurrence of the adverse events that led to discontinuation when switched to zanubrutinib.
“Zanubrutinib provided disease control in more than 95% of evaluable patients who were responding to but intolerant of prior treatment with ibrutinib or acalabrutinib,” he said.
In this longer-term analysis, 67.7% of ibrutinib-intolerance events and 73.0% of acalabrutinib-intolerance events did not recur. These longer-term safety and efficacy outcomes suggest that patients who are intolerant of other BTKi can attain clinical benefit by switching to zanubrutinib.
Conclusion
On the basis of large randomized trials, zanubrutinib now has indications for treatment-naïve CLL and R/R CLL. It joins acalabrutinib as a preferred therapy for these indications, while ibrutinib is now considered a secondary option. The extended follow-up of the SEQUOIA trial reaffirms the sustained efficacy advantage of zanubrutinib as a first-line treatment option. The safety profiles of second-generation BTKi, including zanubrutinib, are more favourable compared to the first-generation ibrutinib. The selectivity and occupancy of BTK by these agents contribute to their safety and efficacy differences. The characteristics that differentiate second- from first-generation BTKi are also potentially relevant to the safety and efficacy differences observed between second-generation agents in independent trials.