Neurology
International Headache Congress 2023
Sustained Suppression of Migraine Demonstrated in DELIVER Follow-up Now Exceeding 18 Months
Seoul – In the treatment and prevention of migraine, the benefit of a monoclonal antibody that targets calcitonin gene-related peptides (CGRP) is durable and extends to patients who have failed multiple prior therapies, according to newly presented long-term data from a multinational trial. Reflecting efficacy and tolerability, the completion rate with 18 months of follow-up was 90% with more than 60% achieving at least a 50% reduction in monthly migraine days (MMD). Notably, benefits were also observed across relevant subgroups affirming the sustained efficacy of the treatment, particularly in patients with a history of medication overuse.
Migraine is an incurable disease that substantially diminishes quality of life and activities of daily living. Despite a broad range of treatments with disparate mechanisms of action, long-term control has been challenging. Unlike traditional therapies, such as anti-inflammatories, antiseizure drugs, vasodilators, pain relievers, and ergots, CGRP-targeted therapies were specifically developed for migraine control. Data with the CGRP-targeted monoclonal antibody eptinezumab, which achieves steady state pharmacokinetics after a single dose, demonstrates that drugs in this class are beneficial for migraine prevention.
Presented at the International Headache Congress (IHC) 2023, new analyses from the DELIVER trial, which was published in Lancet Neurology more than 1 year ago, eptinezumab continued to suppress migraine at 18 months “through multiple measures of control and across multiple relevant subgroups,” reported Dr. Messoud Ashina, Danish Headache Centre, Rigshospitalet Glostrup, University of Copenhagen, Denmark.
>90% of Patients Complete 18 Months of Therapy
Of the 865 patients evaluated at the end of 24 weeks in DELIVER, 782 (90.4%) completed an additional 48 weeks of follow-up. This included almost all patients randomized to eptinezumab and most of those initially randomized to placebo, who were switched to eptinezumab for the study extension. Participants were randomized and followed at more than 90 study sites in Europe and North America.
Like earlier multicenter placebo-controlled phase 3 trials with eptinezumab, such as PROMISE-2, which was published in 2020, the phase 3b DELIVER trial associated eptinezumab with highly significant reductions in MMD relative to placebo. The importance of the update of DELIVER, which was presented at IHC 2023, is that there appears to be no loss of efficacy over follow-up so far, which now extends to 18 months.
In DELIVER, adult migraine patients were enrolled if they had documented evidence of prior treatment failure, whether due to lack of efficacy or intolerability, on at least two migraine therapies from different drug classes. They were randomized in a 1:1:1 ratio of 100 mg of eptinezumab, 300 mg of eptinezumab, or a matching placebo administered intravenously every 12 weeks.
Long-term Effectiveness Observed in Patients with Prior Failed Therapy
For entry into DELIVER, lack of efficacy was defined as no meaningful clinical improvement after at least 3 months on a recommended dose of migraine therapy. Almost all patients (99%) had at least one treatment failure for lack of response. Nearly half had at least one failure for intolerability. Nearly 40% of the study population had experienced at least 3 prior drug failures.
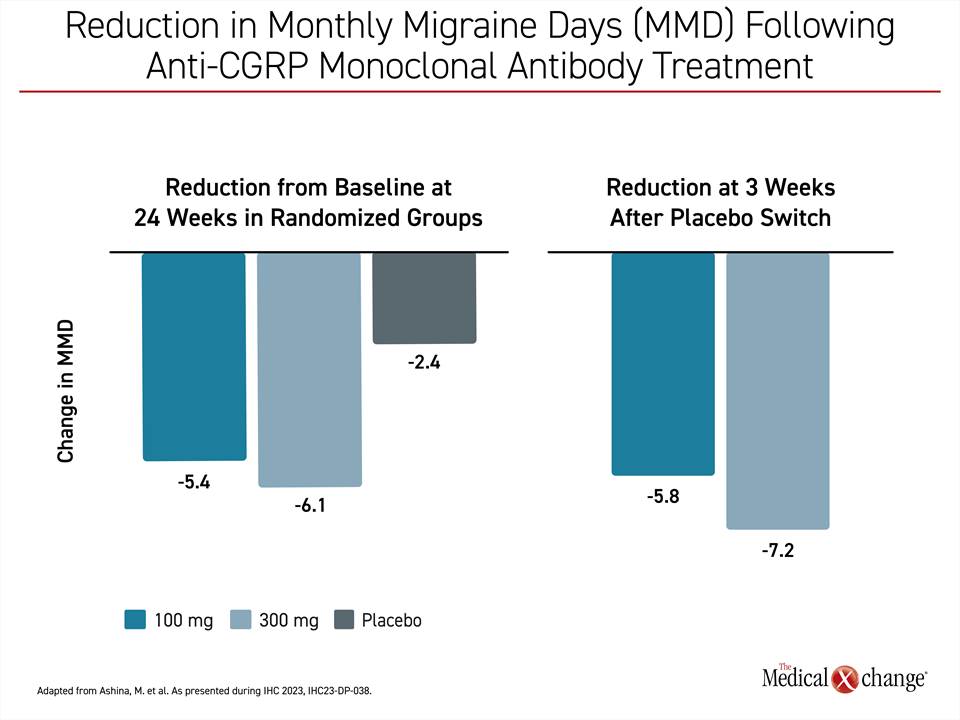
Consistent with other migraine trials, there was a substantial placebo response, but active therapy was more than twice as effective on the primary endpoint with either eptinezumab dose relative to placebo. The advantage over weeks 1-12 for the 100-mg dose (mean 4.8-day reduction) and 300-mg dose (mean 5.3-day reduction) relative to placebo (mean 2.1-day reduction) was highly statistically significant (P<0.0001 for both).
In the extension study, patients previously taking placebo were switched to either 100 mg or 300 mg of eptinezumab. When evaluated three weeks after the switch, the mean reduction in MMD was 5.8 days on the 100-mg dose of eptinezumab and 7.2 days on the 300-mg dose (Figure 1). The reductions, which were sustained over the course of the study extension, confirm that the onset of action was rapid.
Majority Achieved ≥50% Migraine Reduction
“Overall, at the end of 18 months of follow-up, more than 60% achieved at least a 50% reduction in MMD from baseline and more than 30% achieved at least a 75% reduction,” Dr. Ashina reported.
In DELIVER, like the earlier phase 3 trial PROMISE-2, eptinezumab was well tolerated with rates of treatment-emergent adverse events of about 40% observed in those randomized to either dose of eptinezumab or placebo. The types of adverse events were also similar in the active treatment and placebo arms. Serious adverse events were seen in 2% of those treated with eptinezumab and 1% of those randomized to placebo. Anaphylaxis was reported in 2 patients (<1%) randomized to the 300-mg dose of eptinezumab.
The advantage for eptinezumab relative to baseline for the primary outcome was supported by multiple measures of benefit, including symptom reductions based on the Headache Impact Test (HIT-6), a reduction in migraine severity by patient-reported outcomes (PRO), and significant reductions in acute headache medication use (AHM).
Benefit Similar in Medication Overuse Subgroup
In a DELIVER post hoc analysis presented at the IHC, Dr. Anna Gryglas-Dworak, MIGRE Polish Migraine Center, Wroclaw, Poland, reported that the reductions from baseline in headache medications were highly statistically significant whether evaluated for the full dataset or for those who entered the study with medication overuse (P<0.0001 for both). When AHM was evaluated among patients initially randomized to placebo and then switched to eptinezumab, the same phenomenon was observed over the subsequent 48 weeks of therapy.
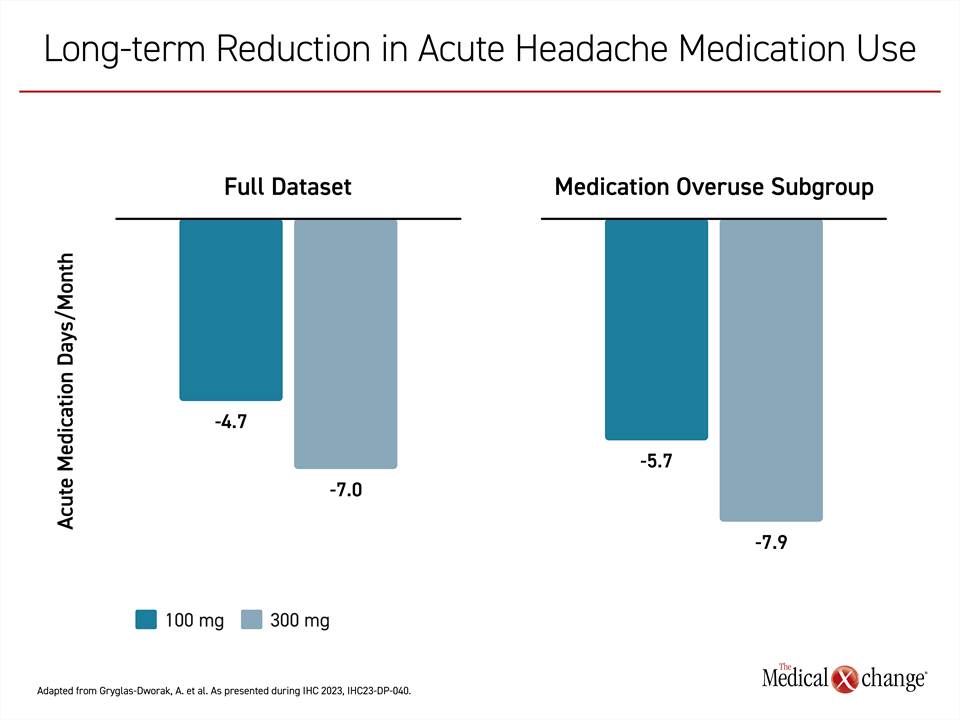
“In the full dataset of placebo patients, the reduction in days per month of AHM was 4.6 and 4.8 over the first 4 weeks for the 100-mg and 300-mg doses respectively. For the subgroup of patients with medication overuse at baseline, the reductions were 6.5 and 6.6 days, respectively,” she reported. “All treatment arms sustained or further reduced AHM use across 18 months of treatment,” she added.
“All treatment arms sustained or further reduced AHM use across 18 months of treatment”
Specifically, the mean AHM use in the 100-mg eptinezumab group was reduced at 72 weeks from baseline by 4.7 days in the full dataset and by 7.0 days in the medication overuse subgroup. In the 300-mg group, the mean reductions were 5.7 and 7.9 days, respectively (Figure 2). These reductions were observed across simple or combination analgesics, ergots, and other classes of migraine therapies, but Dr. Gryglas-Dworak said that the largest reduction in AHM involved triptans.
Acute Migraine Medication Use Reduced
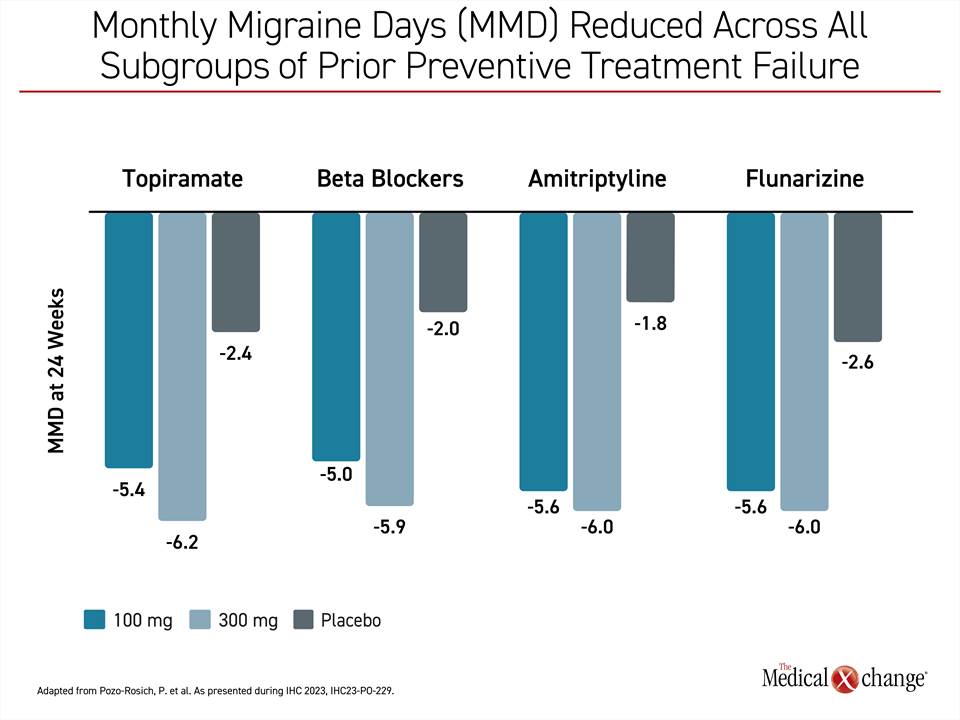
According to another DELIVER post-hoc analysis presented at the IHC, the ability of eptinezumab to reduce MMD relative to placebo was independent of class of prior failed migraine therapy. In this analysis, presented by Dr. Patricia Pozo-Rosich, Headache Unit, Vall d’Hebron University Hospital, Barcelona, Spain, the data were stratified by history of prior failure and response was assessed at week 12 and week 24.
The data were remarkably consistent. Whether patients had previously failed topiramate (633 patients), beta blockers (538 patents), amitriptyline (508 patients), or flunarizine (333 patients), the mean reduction in MMD was 2.6 days or fewer for placebo patients at both timepoints, but ranged between 4.8 and 5.0 days at 12 weeks for the 100-mg dose of eptinezumab and 5.5 to 6.0 days for the 300-mg group for each of these drug classes. At 24 weeks, the reductions ranged from 5.0 to 5.6 days for the 100-mg dose and from 5.9 to 6.2 days for the 300-mg dose (Figure 3).
Other DELIVER Subgroup Studies Support Benefit
The data at the IHC are consistent with other subanalyses that have been published or presented since publication of the 24-week results. In a subgroup DELIVER analysis presented at the Canadian Neurological Sciences Federation held a few months before the IHC, the efficacy of eptinezumab was found to translate directly into major clinical benefits as judged by patients. For the PROs Patient Global Impression of Change (PGIC) and Migraine-specific Quality of Life Questionnaire (MSQ), and the EQ-5D-5L VAS quality of life questionnaire, mean changes at 24 weeks relative to baseline and placebo were highly statistically significant for both doses of eptinezumab, according to Dr. Peter J. Goadsby, Professor of Neurology, King’s College, London, UK. He characterized data as a ”robust” set of evidence for meaningful improvement in well-being.
Moreover, Dr. Goadsby presented a second set of data showing objective improvement in patient function. Based on the migraine-specific Work Productivity Impairment questionnaire (WPAI:M), which was completed every 6 weeks in DELIVER, patients randomized to either dose of eptinezumab relative to placebo had significantly larger reductions in absenteeism (P<0.05), work productivity loss (P<0.001), and activity impairment (P<0.001) as well as improved presenteeism (P<0.001).
“The improvement in work productivity relative to baseline was documented at the first assessment on therapy at 4 weeks and then persisted throughout the study,” Dr. Goadsby reported.
“The improvement in work productivity relative to baseline was documented at the first assessment on therapy at 4 weeks and then persisted throughout the study”
CGRP Inhibition Is a Fundamental Migraine Target
While all four monoclonal antibodies targeting CGRP now approved in Canada—eptinezumab, galcanezumab, fremanezumab, and erenumab—have completed extension studies, the DELIVER trial data was derived from eptinezumab, the only long-acting injectable agent in this class. Eptinezumab blocks both the alpha and beta ligands from binding to CGRP receptors. Unlike oral formulations, intravenous administration of eptinezumab achieves peak plasma concentrations within 60 minutes of administration. A half-life of 28 days permits dosing on an every-3-month schedule, meaning a sustained prophylactic effect often difficult to achieve on oral therapies that are dependent on daily compliance.
According to Dr. Ashina, these characteristics are likely to explain a large and sustained benefit in DELIVER despite enrolling a population that had responded poorly to prior treatments.
The reduction in the primary endpoint of MMD “was consistent across demographic subgroups, migraine classifications, history of medication overuse, and number of prior preventative treatment failures,” Dr. Ashina reported.
“Efficacy was consistent across demographic subgroups, migraine classifications, history of medication overuse, and number of prior preventative treatment failures”
Conclusion
Migraine, one of the most important sources of disease-related disability, is a neurological disease that has been difficult to control despite a large number of therapies in multiple drug classes. While response rates are often substantial to any single therapy, long-term control has been elusive even with drug combinations, often leading to an overuse syndrome. The CGRP-targeted monoclonal antibodies appear to target a fundamental pathway of migraine pain. This is supported by the long-term DELIVER extension data that demonstrated sustained efficacy regardless of prior preventive migraine treatment failure.