Cardiology
15th World Stroke Congress 2023
Reversal Agent for Factor Xa Inhibitors Mitigates Intracranial Bleeding in Phase 4 Trial
Toronto – Intracranial hemorrhage (ICH) associated with factor Xa (FXa) inhibitor anticoagulants, such as apixaban or rivaroxaban, can be controlled with a specific reversal agent in the majority of cases, according to results of a phase 4 multinational latebreaker trial. The trial was terminated early when an interim analysis provided overwhelming evidence that the reversal agent prevents expansion of the hematoma. This reversal agent is already approved in Canada for rapid reversal of FXa inhibitors (rivaroxaban or apixaban) in life-threatening or uncontrolled bleeding. Nonetheless, it is crucial to emphasize the significance of this trial, as it is the first prospective, randomized trial evaluating the efficacy and safety of a specific reversal agent compared to usual care, including prothrombin complex concentrate (PCC) products, in FXa inhibitor-related ICH.
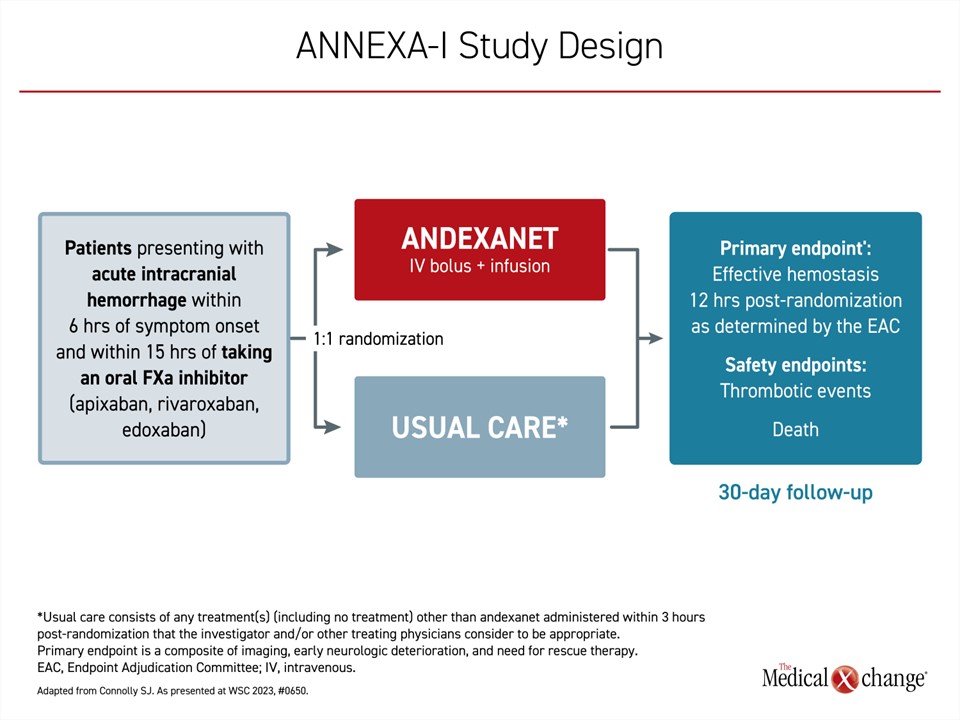
In this multinational phase 4 trial, called ANNEXA-I, patients were randomized to the reversal agent, andexanet alfa or to usual care. The primary endpoint was effective hemostasis 12 hours after randomization. Effective hemostasis was based on imaging and clinical findings. The criteria were: 1) hematoma expansion of ≤20% (excellent) or ≤35% (good) at 12 hours; 2) change in National Institutes of Health Stroke Scale (NIHSS) score of <7 points at 12 hours; and 3) no rescue therapy 3 to 12 hours after randomization. When those randomized to the andexanet alfa group were compared to usual care, which included PCCs in 86.9% of patients, the advantage of the reversal agent was both statistically significant (63.9% vs. 52.4%; P=0.008) and clinically meaningful based on an 11% absolute increase in the proportion of patients with effective hemostasis.
For an exclusive interview with Dr. Andrew Demchuk on the impact to clinical practice, click here
Simply stated, the data prove that “andexanet can be considered for patients with acute ICH associated with FXa inhibition,” according to Dr. Stuart J. Connolly, professor emeritus, Department of Medicine, McMaster University, Hamilton, Ontario.
“[The reversal agent] can be considered for patients with acute ICH associated with FXa inhibition.”
Broadening the Evidence on ICH
The ANNEXA-I trial adds to a body of existing evidence. Andexanet alfa rapidly restores the function of FXa when patients develop a bleeding complication associated with anticoagulants that function by inhibiting FXa. This specific reversal agent acts directly on the target rather than indirectly on other points in the coagulation cascade. Designed as a modified recombinant inactive FXa to bind and sequester FXa inhibition, andexanet alfa rapidly restores the coagulation pathway, including thrombin generation.
The ANNEXA-I trial had a planned enrollment of 900 patients. Patients admitted for ICH within 6 hours of symptoms and 15 hours of taking a dose of a FXa inhibitor were randomized in a 1:1 ratio to andexanet alfa (bolus dose and then infusion) or to usual care (Figure 1). Baseline characteristics were well-balanced between the two groups, with an average age of around 79 years and nearly 45% of female participants. Atrial fibrillation was a common indication for oral anticoagulant use, occurring in 80-90% of the patients. The median baseline hematoma volume was approximately 10 mL.
The trial design called for an interim analysis after half of the planned enrollment had been completed. If at that point there was a significant advantage of the reversal agent over usual care at the predefined statistical power of P<0.031, the trial was eligible for termination for efficacy. When the interim analysis was performed at the end of May of this year, the criterion was met, and the Data Safety Monitoring Committee (DSMB) recommended study termination. After the data lock, the final analysis included 530 patients and was performed just weeks prior to being presented at the 15th World Stroke Congress (WSC) in October.
Advantage Linked to Excellent Response
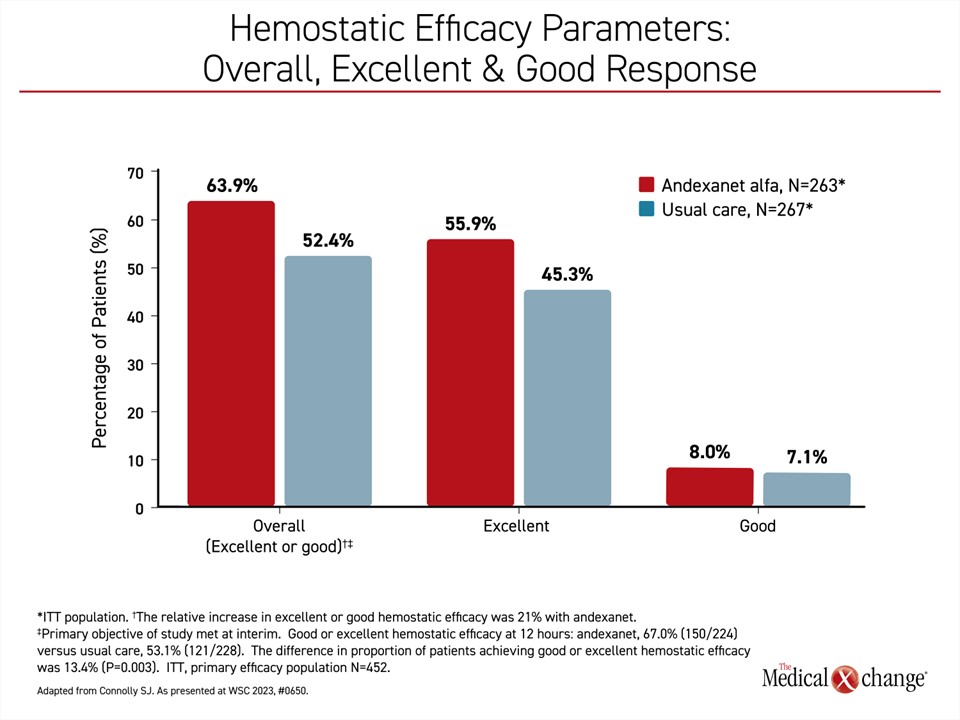
When stratified by good or excellent hemostasis, the analysis showed that the benefit of andexanet alfa over usual care was dominated by the excellent (≤20%) containment of hematoma (55.9% vs. 45.3%) rather than good (≤35%) responses (8.0% vs. 7.1%), emphasizing a rapid onset of action (Figure 2).
“Only a small number of patients fell into the good category [in either arm],” Dr. Connolly reported. “So, most of the benefit of andexanet was quite a substantial benefit.”
“Most of the benefit of the [reversal agent] was quite a substantial benefit.”
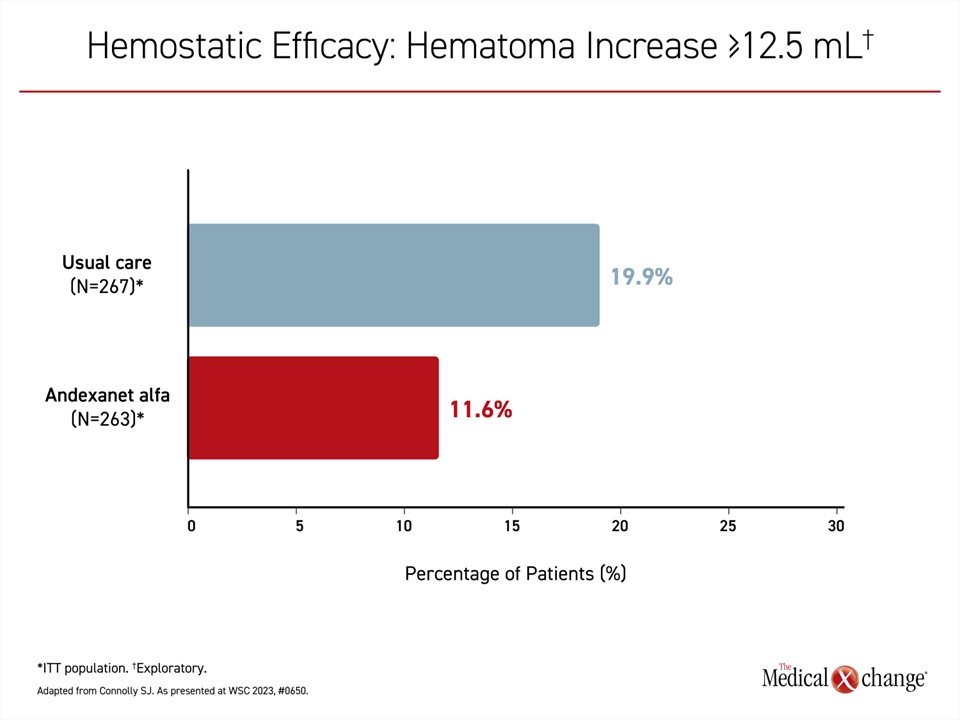
Other points of comparison also confirmed the ability of the reversal agent to contain ICH. For example, when the proportion of patients with a hematoma increase of ≥12.5 mL
was compared, the rate was lower (11.6% vs. 19.0%) in the andexanet alfa arm (Figure 3). When differences in the primary endpoint were compared across multiple variables, such as patient age, baseline stroke score, baseline hematoma volume, and baseline stroke risk score (CHA2DS2-VASc), the effect size for andexanet alfa over usual care was consistent.
Rapid Reduction in Anti-FXa Activity
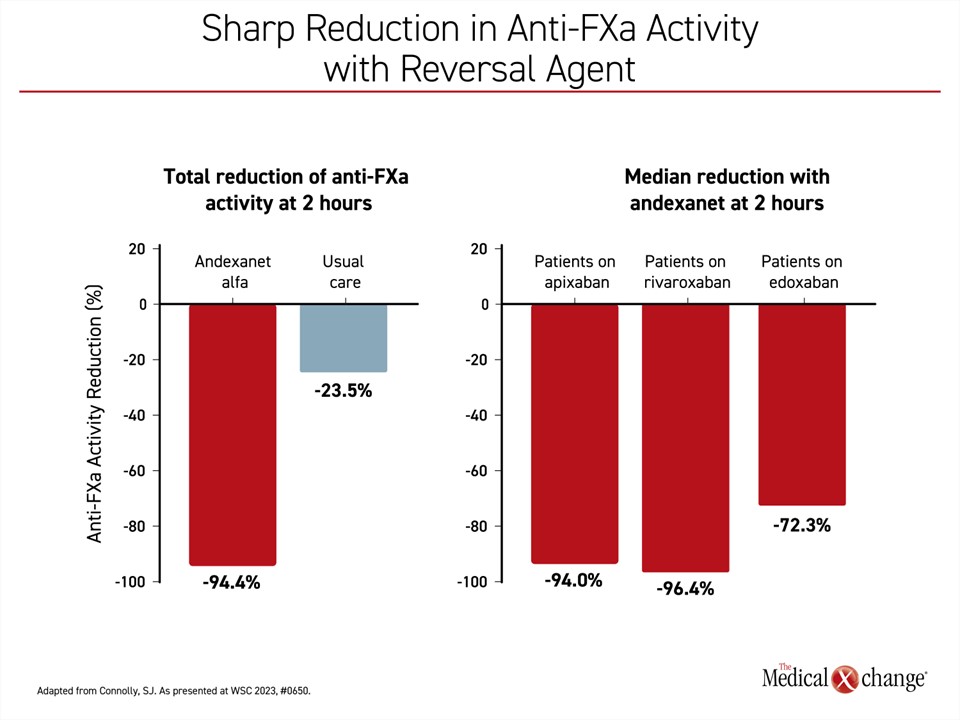
Anti-FXa activity was evaluated as a secondary endpoint. A superior reduction from baseline to nadir (the lowest point) was shown with andexanet alfa versus usual care during the first 2 hours post-randomization (94.4% vs 23.5%; P<0.0001) (Figure 4). Relative to individual FXa therapies, the reduction in anti-FXa activity was similar for apixaban (94.0%) and rivaroxaban (96.4%), and slightly lower for edoxaban (72.3%).
Thrombotic Events Increased
In a population that had been taking oral anticoagulants to prevent thrombotic events, the increased risk of these events in both treatment arms was expected. Overall, these events occurred in low numbers in both groups, but the greater restoration of coagulation in the andexanet alfa arm resulted in a higher rate of events at 30 days, and this difference reached significance (10.3% vs. 5.6%; P=0.048). The most common types of thrombotic events that occurred in the andexanet alfa arm were ischemic stroke and myocardial infarction.
The rate of events did not exceed 5% in either arm, with the exception of ischemic stroke (6.5% vs. 1.5%) among those randomized to andexanet alfa. Conversely, the combined rate of deep vein thrombosis and pulmonary embolism was numerically lower among those randomized to andexanet alfa (0.8% vs. 1.0%). There was no significant difference in mortality at 30 days (27.8% vs. 25.5%).
The higher number of thrombotic events in the andexanet alfa arm reflects an expected risk from intervening in life-threatening ICH events, according to Dr. Connolly, but the data support a benefit-to-risk ratio favouring andexanet alfa overall and across individual patient subgroups. Presenting data to support the consistency of benefit for reversing FXa activity with andexanet alfa, Dr. Connolly said, “the general message is that we do not see any significant interactions for any of the subgroups. The treatment benefit with andexanet is fairly uniform.”
“The general message is that we do not see any significant interactions for any of the subgroups. The treatment benefit with [this reversal agent] is fairly uniform.”
The balance of benefit to risk is an important consideration in the types of patients who develop ICH associated with oral anticoagulation. In ANNEXA-I, the mean patient age was 79 years. Nearly 90% had atrial fibrillation, and more than 20% had a prior history of stroke. More than 10% had a prior history of myocardial infarction. The baseline NIHSS score was 9.0 and the median CHA2DS2-VASc score was 4.0, signifying a population at high risk of both bleeding and thromboses.
Earlier Phase 3 Study Published in 2023
There have been numerous prior trials of andexanet alfa for reversing FXa inhibitors, including a phase 3b/4 multicenter single-arm trial called ANNEXA-4. The full study cohort was published in the New England Journal of Medicine in 2019. That trial of 352 patients with acute major bleeding associated with FXa inhibitors was not limited to cases of ICH, although these represented the majority (64%) of bleeding events. For the primary endpoint of hemostasis in that trial, which was also led by Dr. Connolly, more than 80% of evaluable patients had good or excellent hemostasis when adjudicated with prespecified criteria.
In a final ANNEXA-4 cohort report published in Circulation earlier this year, 479 patients with acute major bleeding within 18 hours of taking a FXa inhibitor were evaluated by Dr. Truman J. Milling, who has appointments in both the Department of Neurology and the Department of Surgery, University of Texas Dell Medical School, Austin. The primary efficacy endpoint was good or excellent hemostasis at 12 hours defined by prespecified criteria such as hematoma expansion in patients with ICH, bleeding control in GI hemorrhage, drop in hemoglobin/hematocrit, and other measures.
The final analysis reconfirmed excellent or good hemostasis in 80% of patients in the context of acceptable safety. Anti-FXa activity was reduced by 93% among those who had been taking apixaban and 94% among those who had been receiving rivaroxaban. Together, these groups represented 90% of the study population. The reduction in anti-FXa activity for those previously taking edoxaban, which represented <10% of patients, was 71%.
An independent adjudication committee reviewed thrombotic events within a 30-day period, identifying occurrences in 50 patients (10.4%). Notably, 31 cases occurred between days 6-30 following the initiation of reversal therapy, with the remainder manifesting within the first 6 days. The most common events were ischemic strokes (22) and deep vein thromboses (15). During the 30 days, there were no thrombotic events in any of the 130 patients (27.1%) who resumed oral anticoagulation. The mortality rate was 15.7%, but deaths were concentrated in those 75 years of age or greater (19.6%) relative to younger (6.8%).
Benefit-to-Risk Ratio Favours FXa Reversal
Given the overall benefit-to-risk ratio in this study, Dr. Milling confirmed that the data “support the use of andexanet alfa as a specific reversal agent for FXa-associated acute major bleeding.”
“[The phase 3b data] support the use of [this] specific reversal agent for FXa-associated acute major bleeding.”
There are already published data demonstrating that andexanet alfa is associated with better outcomes relative to 4-PCC in patients with major bleeding associated with FXa inhibitors. However, the ANNEXA-I trial makes this the first prospective, randomized trial evaluating the efficacy and safety of a specific reversal agent, including PCCs, in the control of FXa inhibitor‑related ICH. Although idarucizumab is indicated as a reversal agent for adult patients treated with dabigatran, a direct thrombin inhibitor (DTI), for control of life-threatening or uncontrolled bleeding related to DTI, it has not been compared to usual care in a randomized trial for the specific control of ICH.
FXa inhibitors are among the most commonly employed agents to reduce thrombotic events in high-risk patients in Canada and elsewhere. The availability of an antidote provides an important option to manage life-threatening bleeds in patients on anticoagulants.
Conclusion
Making a reversal agent available for urgent use in emergency centers where ICH and other major bleeding events associated with anticoagulants is now a reasonable standard of care. The ANNEXA-I trial has demonstrated that prompt initiation of the reversal agent andexanet alfa achieves effective hemostasis relative to usual care, including PCCs, in patients who present with ICH associated with FXa inhibitors such as apixaban or rivaroxaban.