Hematology
Toronto Complement Conference (TCC) 2024
Paroxysmal Nocturnal Hemoglobinuria: Targeting Unmet Needs with Newest Proximal Complement Inhibitors
Toronto –Treatment of paroxysmal nocturnal hemoglobinuria (PNH) with C5 inhibitors has improved survival and reduced thrombosis, but proximal inhibitors, a newer class of agents to block complement activation, target earlier stages of the complement cascade. In PNH, the data so far suggest that the newer class of agents are associated with less residual anemia caused by extravascular hemolysis (EVH), reduced treatment burden, and improved quality of life. These, as well as other new treatment strategies for this ultra-rare disorder, are already or may soon lead to new directions in PNH management, according to a summary at this year’s Toronto Complement Conference (TCC).
Classical Triad of PNH Symptoms
PNH is an ultra-rare acquired non-malignant clonal abnormality of hematopoietic stem cells caused by a mutation on the X chromosome, leading to the loss of GPI anchor-linked complement regulatory molecules CD55 and CD59, explained Dr. Christopher Patriquin, Assistant Professor of Medicine and Clinical Investigator, University of Toronto, and Consultant, Hematology & Apheresis Medicine, University Health Network.
Without these anchor proteins, there is cumulative deposition of C3 and its split products, driving terminal complement activation and causing deposition of membrane attack complex on the cell surface.
“This is the natural history of the disease that we see, leading to intravascular hemolysis because of this occurring on the red cells, but also an increased risk of thrombosis because this same process can happen on the surface of our monocytes, our neutrophils and our platelets.”
For an exclusive interview with Dr. Christopher J. Patriquin on the impact to clinical practice, click here
PNH presents with a “classical triad” of thrombosis, DAT-negative hemolysis, and bone marrow failure, but many of the symptoms are nonspecific. Symptoms associated with PNH are due to intravascular hemolytic activity, Dr. Patriquin said.
“Hemoglobin is great inside the cell, but very dangerous outside,” acting as an avid consumer of nitric oxide. This causes a variety of downstream effects, some of which have a direct impact on survival – arterial and venous thrombosis and renal failure – but that also affect quality of life. Fatigue is a widespread physical symptom reported by patients and is often debilitating, but symptoms vary and may include hemoglobinuria, dyspnea, dysphagia and erectile dysfunction.
“Hemoglobin is great inside the cell, but very dangerous outside.”
CATCH: Keeping a High Level of Suspicion for Diagnosis
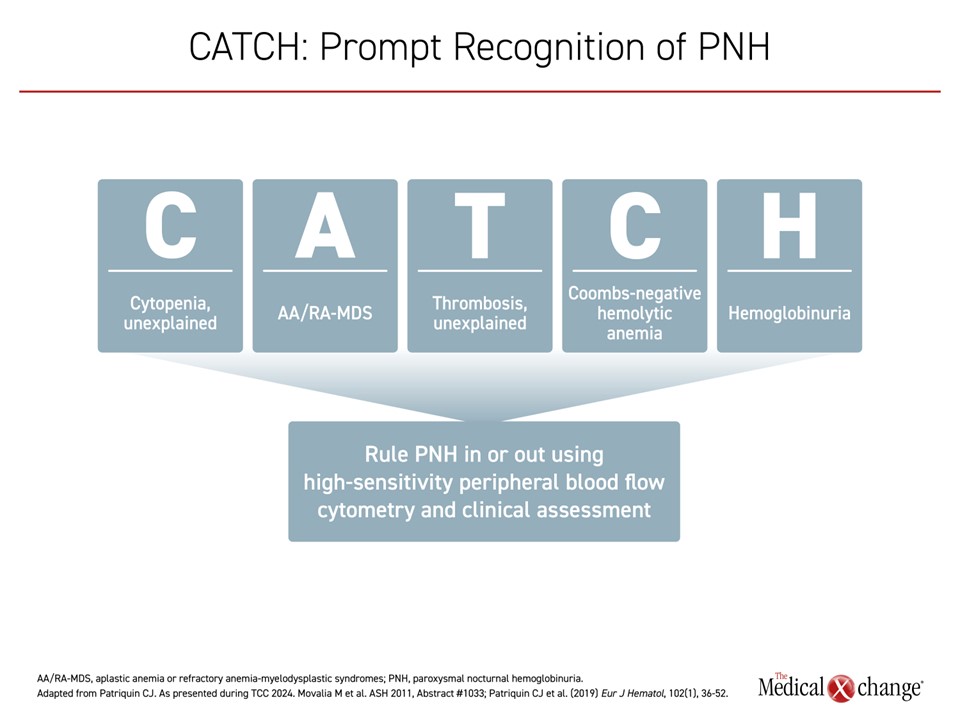
To make a diagnosis of PNH, as with any rare disease, “you have to think about it,” said Dr. Patriquin. He suggested using a mnemonic that has been endorsed by the Canadian PNH Network to underline clinical features that may prompt physicians to test for PNH. CATCH stands for unexplained Cytopenias, Aplastic anemia or refractory anemia-myelodysplastic syndromes (AA/RA-MDS), unexplained Thrombosis, Coombs-negative hemolysis, and Hemoglobinuria (Figure 1). “The good news,” he continued, “is once a diagnosis is suspected, high-sensitivity flow cytometry is readily available in Canada, both at academic institutions and community labs”.
It is important to recognize the patients and start them on appropriate treatment. Natural history data show
5-year mortality associated with PNH is 35%. Continuous complement inhibition in the right patient is “really crucial to improving their outcomes,” he explained.
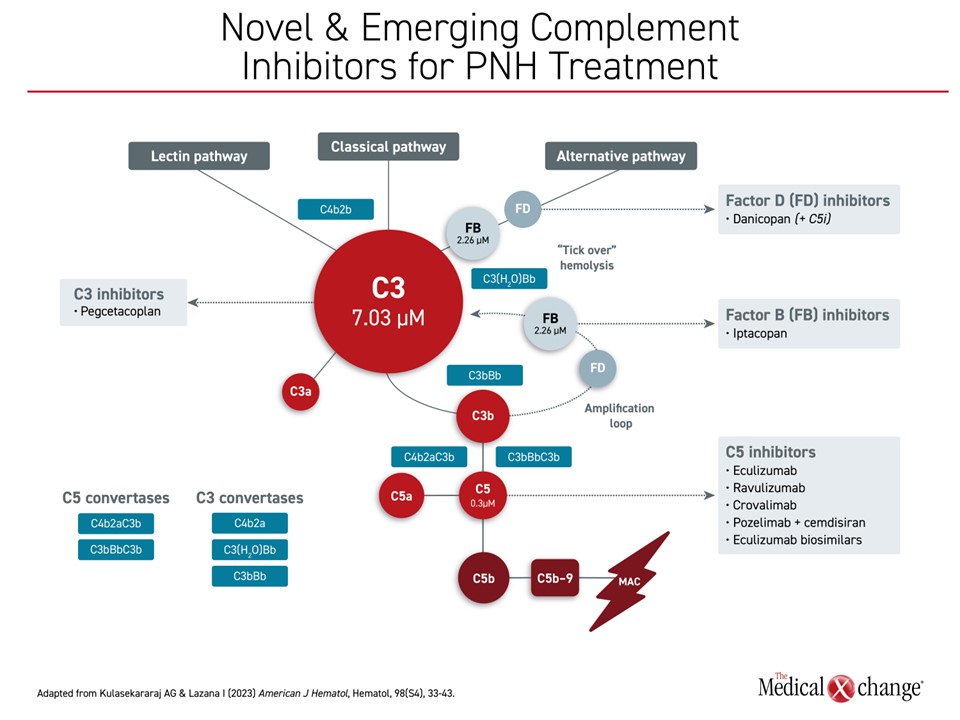
A Closer Look at Complement Inhibitors Already Available or Under Development in PNH
Several agents for the treatment of PNH are in Phase 3, completed Phase 3, or have been approved in Canada (Figure 2). Intravenous anti-C5 monoclonal antibodies became the standard of care for PNH with Health Canada approval of eculizumab in 2009, based on study results that showed intravascular hemolysis control and improved long-term survival relative to placebo. The longer-acting ravulizumab, which requires administration every 8 weeks instead of every 2, showed similar efficacy when introduced subsequently.
However, due to unmet needs following the introduction of C5 inhibitors, particularly the anemia that persists in some patients, proximal complement inhibition now seems likely to assume a growing role in PNH, according to data presented by Dr. Patriquin.
“Due to unmet needs following the introduction of C5 inhibitors, proximal complement inhibition now seems likely to assume a growing role in PNH.”
The exact role of proximal complement inhibitors in PNH is still being evaluated. The C3 inhibitor pegcetacoplan, which is administered by subcutaneous injection, and danicopan, used as an add-on oral therapy to a C5 inhibitor, are already approved for PNH in Canada.
In the 12 week interim analysis of the ALPHA trial, the factor D inhibitor danicopan improved hemoglobin concentrations relative to placebo when used as an add-on therapy to eculizumab or ravulizumab. Results showed benefit in patients with PNH who experienced clinically significant EVH.
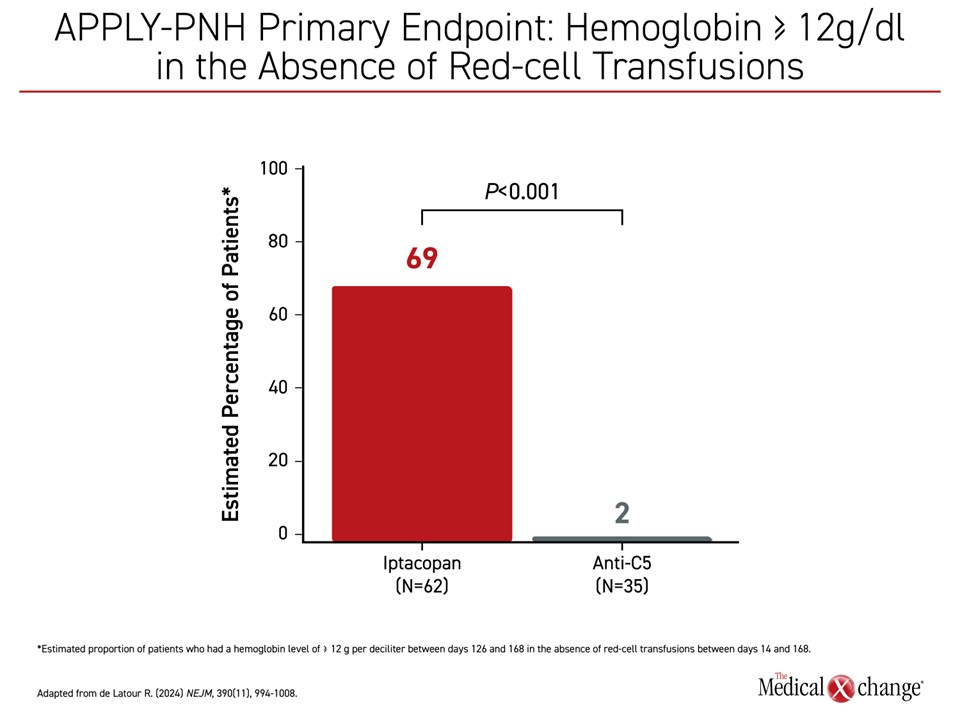
The stand-alone oral proximal complement inhibitor iptacopan is being considered for regulatory approval after favourable results in phase 3 trials. The oral factor B inhibitor was studied in two phase 3 trials published together in the New England Journal of Medicine. In APPLY-PNH, patients were enrolled who had been on a stable regimen of eculizumab or ravulizumab for at least 6 months and still had mean hemoglobin levels <10 g/dL. Despite the inadequate response on C5 therapy, identifying a patient at high risk of PNH complications, 69% of iptacopan patients versus 2% of patients (P<0.001) who remained on a C5 inhibitor achieved hemoglobin levels ≥12 g/dL between days 126 and 168 (Figure 3). The proportion of patients refractory to standard therapy achieving ≥2 g/dl was also dramatically increased (82% vs 2%; P<0.001). Only 5% required transfusion in the iptacopan group versus 40% of those in the placebo group.
In the single-group APPOINT-PNH phase 3 trial, PNH patients naïve to C5 therapy were enrolled if their lactate dehydrogenase (LDH) levels were >1.5 times the upper limit of normal. Although patients did not receive a C5 inhibitor or other PNH-targeted therapy, no patient required transfusion and 31 of 33 patients had a ≥2 g/dL increase in hemoglobin.
Both the APPLY-PNH and APPOINT-PNH results showed similar and consistent tolerability and safety profiles and show oral iptacopan controls hemolysis while nearly eliminating the need for blood transfusions. “What we can see with proximal inhibition in general is almost a class effect of improvement,” said Dr. Patriquin.
“What we can see with proximal inhibition in general is almost a class effect of improvement.”
Comprehensive Control of Intravascular and Extravascular Hemolysis
Even though the three proximal complement inhibitors, pegcetacoplan, danicopan and iptacopan have different mechanisms of action, each inhibits complement at an earlier stage in the complement cascade. It was once thought that this earlier action might increase the risk of EVH, but the opposite has been true. Each has been associated with a reduction in anemia, a benefit that is likely at least in part to be attributed to attenuation of EVH. Of note, robust outcomes were seen for patients treated with iptacopan in the APPLY-PNH study, which included patients with residual anemia, again underlining the promise for practice-changing potential in the treatment of PNH with proximal complement inhibitors.
C5 Inhibitors Demonstrate Improved Survival but Unmet Needs Remain
C5 inhibitors were a breakthrough when they were introduced, but alternatives are needed for those who develop EVH. In clinical studies, eculizumab rapidly reduces levels of LDH, a surrogate marker of intravascular hemolysis, to around the upper limit of normal. This has been associated with improved survival, perhaps largely due to significant reductions in thrombotic events, Dr. Patriquin said. Pregnancy outcomes in PNH patients have also been significantly improved, he reported.
“But there are things we can still aim to do,” he said. First, quality of life for these patients could be improved, and “related to that, we can reduce treatment burden.”
Some of this treatment burden involves frequency and type of drug administration. Both eculizumab and ravulizumab require intravenous administration. Another C5 inhibitor, crovalimab, which has been shown to be non-inferior to eculizumab, is administered subcutaneously every four weeks after a loading period but does not eliminate the need for injection.
Of the proximal complement inhibitors, pegcetacoplan is also administered subcutaneously, but in this case, the schedule is twice-weekly. Danicopan is taken orally however, is approved as an add-on to a C5 inhibitor and therefore, iptacopan is the only potential all-oral therapy for PNH.
Treatment with C5 inhibition should be monitored using LDH as a marker of intravascular hemolysis, and when possible, CH50 as a marker of terminal complement activation, Dr. Patriquin noted. Indicating that C5 inhibitors remain first-line therapies, he suggested a proximal complement inhibitor or one combined with a C5 inhibitor should be considered for patients with inadequate response to C5 treatment after 3 to 6 months, or if they are intolerant to C5 treatment.
If LDH levels remain 1.5 times over the upper limit of normal after treatment, particularly if there is still detectable terminal complement activation on CH50, there should be concern about breakthrough hemolysis, whether due to pharmacokinetic or pharmacodynamic processes or other source of inadequate complement inhibition.
Even if LDH is <1.5 times the upper limit of normal, about 40% of patients still have significant anemia that can be quite symptomatic, and may be caused by EVH, which is a sign of incomplete complement blockade, Dr. Patriquin cautioned.
Looking Towards New Treatment Options to Further Address Challenges
Although it may change in the next few years, Dr. Patriquin reported, right now, the recommendation for treatment of PNH patients is to start with a terminal complement inhibitor, eculizumab or ravulizumab, although other C5 inhibitors may soon be available.
If the patient has an inadequate response in terms of change in hemoglobin after 3 to 6 months, “this is where we would look to other potential therapies to discuss with our patients, and that may be proximal inhibitors,” he added.
“We need to ensure that LDH is below 1.5 times the upper limit of normal, and at least in the data we have so far – and of course everything needs to continue to mature – we’re seeing essentially that,” noted
Dr. Patriquin, referring to proximal inhibitors.
Other data to support the role of proximal complement inhibitors in the treatment of PNH are expected. At the ASH 2024 meeting, for example, an open-label extension of the phase 3 iptacopan trials showed improvement in patient-reported health-related quality of life as well as in investigator-assessed signs and symptoms of disease at the end of 48 weeks.
Overall, counselling sessions with patients are now “much more complex, because we’re going through obviously much of the new data, but we also have to talk about their preferences for various routes of therapy,” he added. “Pegcetacoplan is given twice weekly subcutaneously, but they can do that on their own. Oral therapies are given twice daily with iptacopan, or danicopan three times a day with an ongoing C5 backbone.”
Conclusion
PNH presents with common and non-specific symptoms and deserves “a high index of suspicion and a low threshold for testing,” according to Dr. Patriquin. While C5 inhibitors are effective for PNH, they can unmask EVH that is clinically meaningful in some patients.
PNH deserves “a high index of suspicion and a low threshold for testing.”
Proximal complement inhibitors address these limitations by targeting earlier in the complement cascade, offering improvements in anemia, reduced transfusion requirements, and enhanced quality of life. Therapies such as pegcetacoplan, danicopan, and iptacopan provide new avenues for comprehensive hemolysis control, with stand-alone oral therapies like iptacopan also offering greater flexibility in administration and tailoring treatment to individual needs.
As long-term extension data mature for the newer proximal inhibitors, physicians and patients will have more options to determine which treatments will best meet individual needs in terms of efficacy and safety but one that fits particular disease characteristics and lifestyles