Rheumatology
Psoriatic Arthritis: Expert Review and Commentary from Published Literature
New GRAPPA Treatment Recommendations for Psoriatic Arthritis
Caylib Durand, PhD, MD, FRCPC
Rheumatologist, Peak Medical Rheumatology Clinic
Clinical Lecturer, University of Calgary
Calgary, Alberta
Marie-Anaïs Rémillard, MD, FRCPC
Rheumatology Institute of Montreal
Montréal, Quebec
The Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) has completed and will soon publish new evidence-based treatment recommendations for psoriatic arthritis (PsA). Relative to the previous recommendations published in 2016, the list of first-line therapies for the major domains of PsA has changed. On the basis of controlled trials, two drug classes—tumour necrosis factor inhibitors (TNFi) and interleukin-17 inhibitors (IL-17i)—are now recommended as first-line therapy for moderate-to-severe disease across all six PsA domains. According to an author-led introduction to the treatment recommendations at the European Alliance of Associations for Rheumatology (EULAR) 2021 Annual Meeting, controlled trials have also led to the inclusion of other targeted therapies, such as Janus kinase inhibitors (JAKi), although their indications across disease domains are more limited.
Background
PsA will eventually develop in about one-third of patients with psoriasis.1 On average, joint involvement follows skin lesions by 10 years, but this pattern is not consistent. In about 15% of patients, the onset of skin and joint manifestations develops within a similar timeframe.1 Six domains of PsA are recognized; in addition to psoriatic skin, these are peripheral arthritis, axial disease, enthesitis, dactylitis, and nail manifestations.2 In the province of Ontario, a 2015 population-based study conducted in adults (age ≥20 years) found a psoriasis prevalence of 2.32% and a PsA prevalence of 0.15%.3 The authors of this study characterized these prevalence rates along with the observed male-to-female ratio of 1:1 as similar to that reported in the US and Europe.
For an exclusive interview with Dr. Caylib Durand on the impact to clinical practice, click here
PsA is an autoimmune inflammatory process that has been and continues to be managed primarily with therapies that modify immune system activity.4 Several interrelated pathways resulting in activated lymphocytes and cytokine release are common to both skin and joint manifestation. In any individual patient, the disease activity within the six domains of PsA can vary substantially. Likely influenced by both genetic and environmental factors, the severity of symptoms in one domain is not necessarily reflected in others, meaning there can be significant activity in some but little or no involvement in others. Moreover, individual patients do not necessarily tolerate similar degrees of activity in a given domain in the same way. Optimal treatment depends on evaluating activity in each of the disease domains and then judging treatment response on both objective terms and on the basis of patient experience, including subjective measures of pain control, function, patient satisfaction and quality of life.
Many of the traditional disease-modifying antirheumatic drugs (DMARDs) employed for other autoimmune diseases with joint involvement, including non-steroidal anti-inflammatory drugs (NSAIDs), corticosteroids, methotrexate and biologics, are effective in PsA.5 In the forthcoming GRAPPA recommendations, an order of treatment has been described for each PsA domain,6 as was employed in the 2016 treatment recommendations.7 Building on previous recommendations, the evidence-based changes in these upcoming treatment schemes are drawn from data published since 2016. This includes pivotal trials with newly-approved therapies as well as new controlled evidence with biologics available at the time that the last set of treatment recommendations were published.
Yet, the overarching principles for treatment goals have not changed. These include control of pain, prevention of structural damage, optimizing functional capacity, and achieving an acceptable quality of life. The new recommendations, like the previous set, also urge evaluation and treatment of comorbidities common to PsA. Control of these comorbidities, such as concomitant autoimmune diseases, obesity, metabolic syndrome, diabetes, liver disease, cardiovascular disease, uveitis, and mood disorders, contribute to optimizing functional capacity and quality of life. Due to the demands imposed by symptoms and comorbidities involving multiple organ systems, the treatment recommendations advocate a multidisciplinary approach to PsA management.
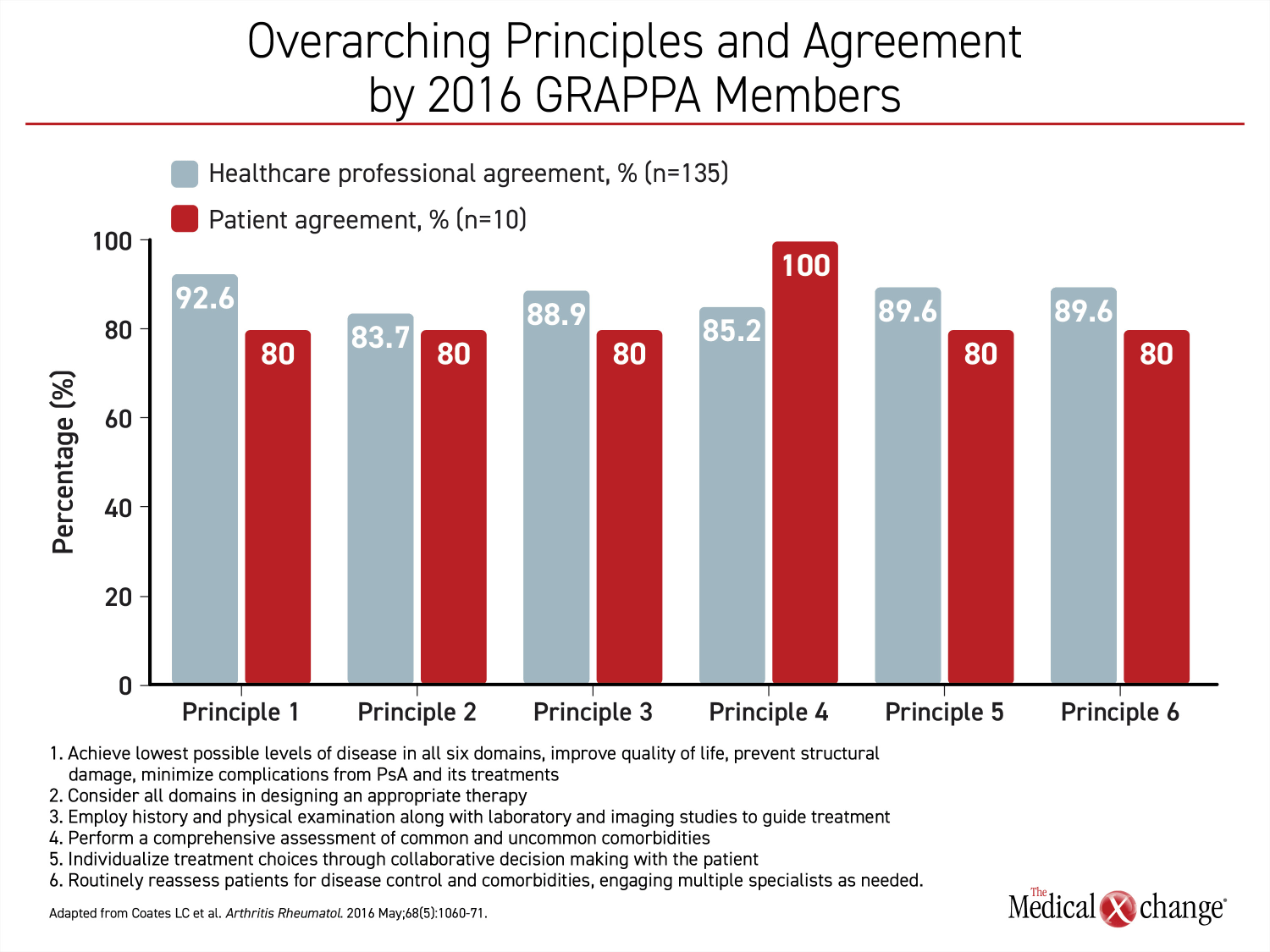
In the voting for the overarching principles of PsA treatment for the 2016 recommendations, a comprehensive approach was embraced by both healthcare professionals and patient representatives collaborating on developing that document (Figure 1).
New GRAPPA Treatment Recommendations: Shifting Focus towards Moderate-to-Severe Cases
The need for revised recommendations was generated by practice-changing trials and studies published over the past 5 years, according to the GRAPPA writing committee.6 In a review of the medical literature in PsA over this period, the expert panel assembled to produce the revised treatment recommendations and employed the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach. The panel included dermatologists, rheumatologists, patient advocates, and experts in related comorbidities, such as gastroenterologists and ophthalmologists. Based on their specific expertise, the panel members were assembled into groups to identify optimal practice for clinical management in each of the PsA domains. Final voting by all panel members produced recommendations that were identified as “strong” or “conditional” based on the quality of the data.
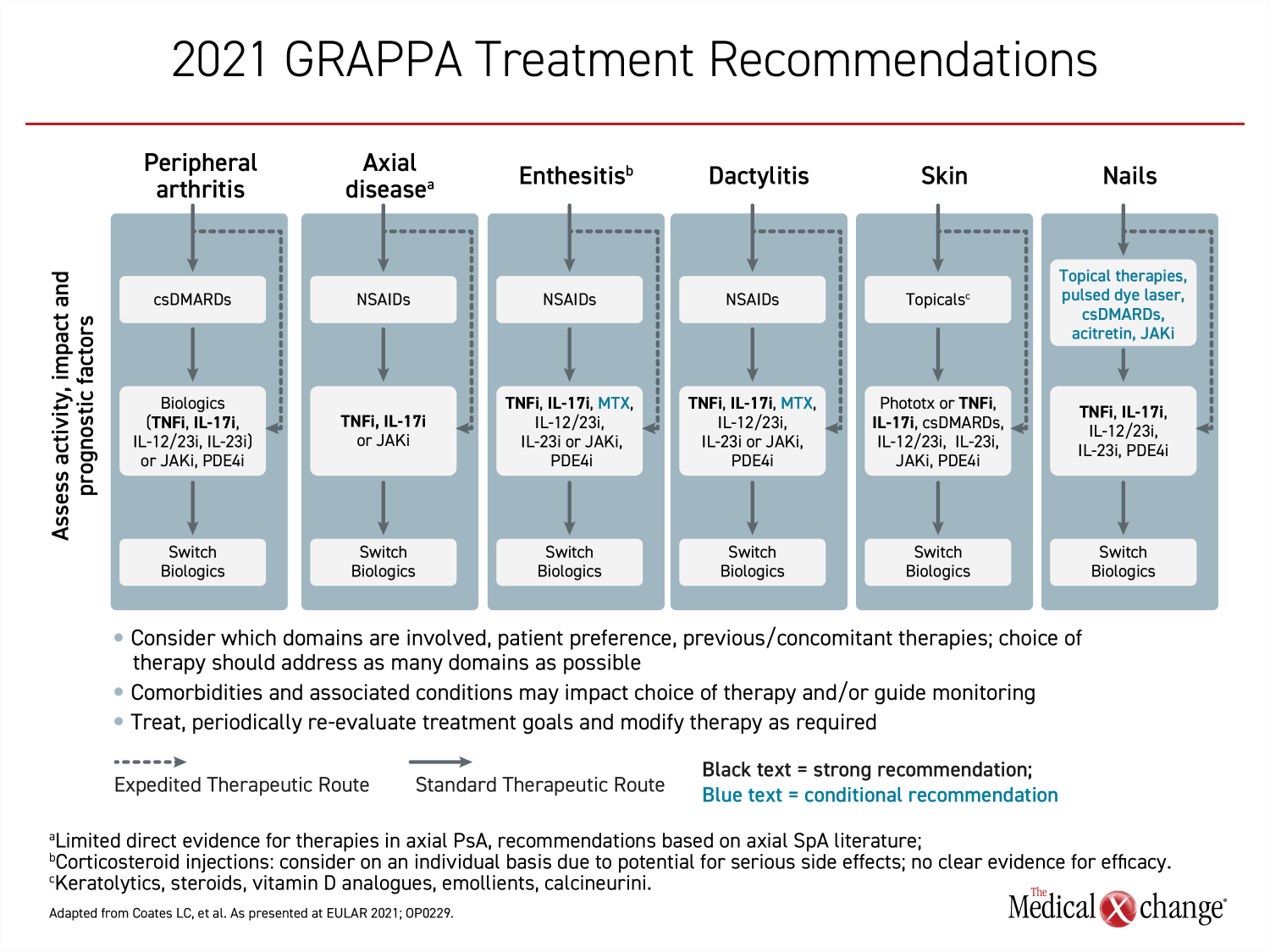
Relative to the previous recommendations, the strategies to control mild symptoms in each of the six domains have not changed. For patients with axial disease, enthesitis and dactylitis, NSAIDs remain an appropriate first-line option. For peripheral arthritis, conventional synthetic DMARDs (csDMARDs), such as methotrexate, are preferred. For skin lesions, topical therapies, such as corticosteroids, should be used first for mild disease. Corticosteroids, acitretin, and JAKi in topical preparations along with csDMARDs and pulsed dye layers are the recommended options for mild nail involvement. In all cases, the suitability of first-line therapies is determined by response and tolerability.
The revisions, therefore, are concentrated in the management of moderate-to-severe symptoms. These revisions reflect new therapies approved for PsA on the basis of controlled trials as well as expanded indications for therapies included in the 2016 recommendations. Unlike the 2016 treatment recommendations, biologics or targeted therapies now have a strong recommendation for the management of moderate-to-severe symptoms in all six domains. However, the specific biologics and targeted therapies appropriate for each domain differ.
Where biologics were recommended first-line in the 2016 recommendations, the options included TNFi, IL-17i or IL-12/23i. In the new set of treatment recommendations, TNFi and IL-17i remain among first-line therapies for those domains in which biologics had been recommended previously, and are the only treatments recommended across all six domains. The difference is that other biologics and other targeted therapies have now joined TNFi and IL-17i in each of the domains. Of biologics and targeted therapies, only TNFi and IL-17i have gained “strong” recommendation status across all six domains in the new treatment recommendations (Figure 2).
The broad efficacy of TNFi and IL-17i in PsA is relevant to drug selection. As an overriding principle, the GRAPPA treatment recommendations encourage the selection of therapies that address as many domains as possible. Among other advantages in patients with significant disease involvement, therapies active against multiple disease domains reduce the burden of treatment. Relative to the 2016 GRAPPA recommendations, IL-12/23i has been added as an option in the domain of skin involvement in patients not adequately controlled on a topical agent. It is not included as a first-line option for moderate-to-severe axial disease activity which is changed from the 2016 recommendations. Phosphodiesterase-4 inhibitors (PDE-4i), which were considered acceptable options in four domains of moderate-to-severe PsA in 2016, are now included in five domains.
There were no agents within the JAKi class of targeted agents approved for PsA at the time that the last treatment recommendations were published. Tofacitinib, which showed efficacy in a pivotal phase 3 trial that enrolled patients with an inadequate response to TNFi,8 was approved in 2017. Subsequently conducted clinical trials with other JAKi, including baricitinib and upadacitinib,9,10 have also shown activity in PsA although regulatory approvals differ by country. JAKi are considered an option for moderate-to-severe PsA in five domains.
There were no selective inhibitors of IL-23 included in the previous recommendations. The first IL-23i approved for PsA, guselkumab, which binds to the p19 subunit of the cytokine, demonstrated efficacy in a pivotal phase 3 trial published in 2020 that enrolled both TNFi-experienced and biologic-naïve patients with PsA.11 In the forthcoming GRAPPA recommendations, that treatment, like IL-12/23i, has been added as an option for moderate-to-severe disease in five of the six domains but is not considered suitable for axial disease.
First Choice Biologic: Weighing Options
Inhibitors of the TNF cytokine, which have indications for autoimmune inflammatory diseases across multiple organ systems, established a role for biologics targeting pathways of immune signalling. In PsA as in other chronic diseases driven by dysregulated immune activity, benefit has now been established for biologics targeting a range of cytokines as well as for targeted therapies that provide symptom control by interrupting pathologically upregulated inflammatory pathways. Despite a limited number of direct comparisons, these therapies are not considered interchangeable across disease domains on the basis of available evidence in the new or previous GRAPPA treatment recommendations. The relative differences might be best understood by comparing TNFi to IL-17i, the only drug classes recommended across each PsA domain. Agents in both drug classes have demonstrated efficacy, but differences across biologics and targeted drugs are exemplified in their activity and safety profiles.
For example, the EULAR updated recommendations of 2019 indicated that IL-17i may be preferred over TNFi for joint symptoms of PsA in those with substantial skin involvement due to the evidence that drugs in the class have greater efficacy against both domains.12 These same recommendations indicated that TNFi may be preferred in patients with comorbid inflammatory bowel disease (IBD) or uveitis. The new GRAPPA recommendations do not identify a treatment preference for comorbid uveitis but also caution against the use of IL-17i in IBD. The prevalence of comorbid PsA and IBD is low, occurring in <1.5% for both ulcerative colitis and Crohn’s disease.13
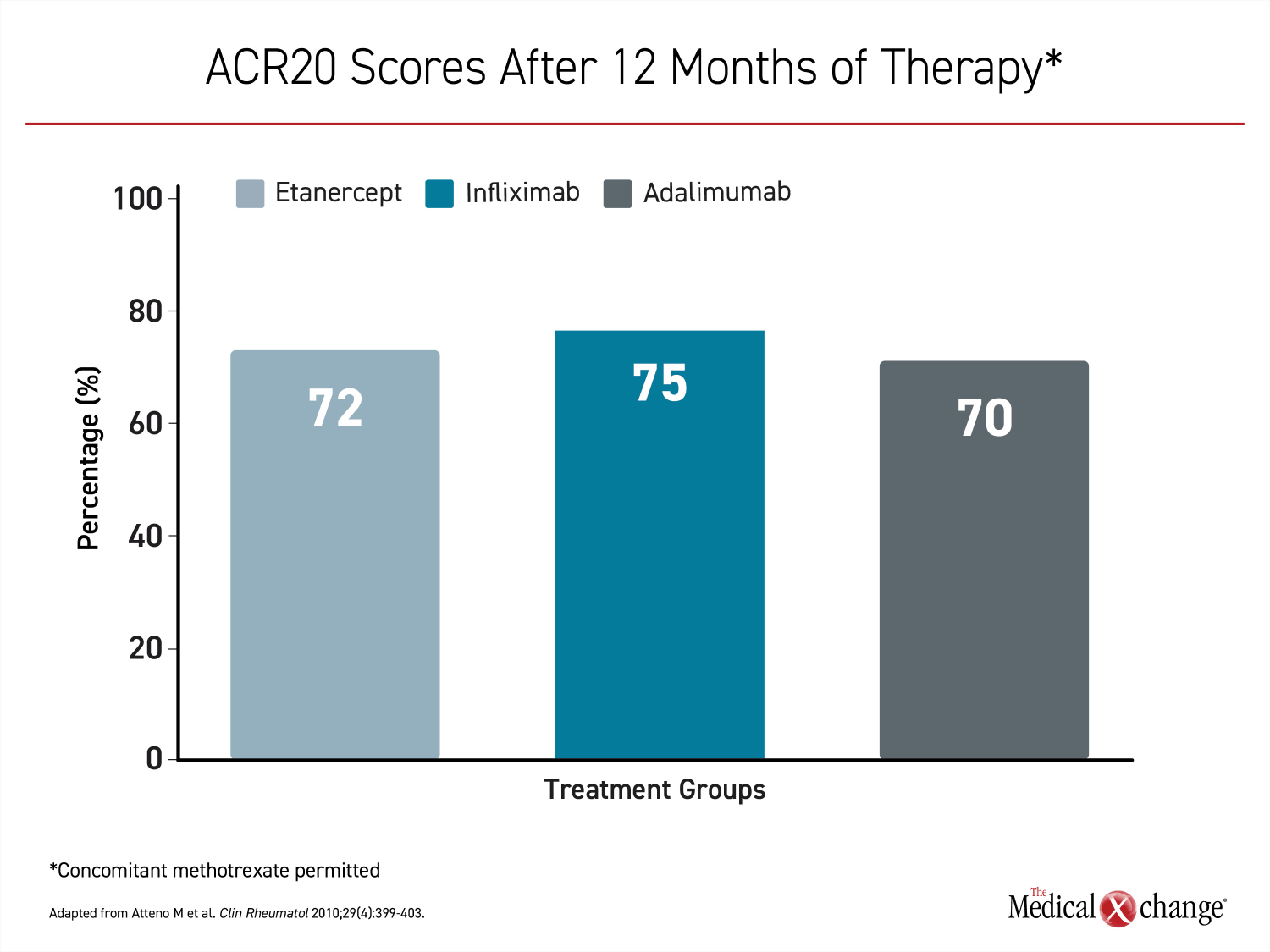
The first TNFi approved for PsA, etanercept, has been available for nearly 20 years.14 It was joined by infliximab and adalimumab in 2005. The efficacy of these therapies appears to be similar.15 In one direct comparison, there were no substantial differences at 12 months in ACR20 scores16 (Figure 3). The same study showed similar results for PASI scores and quality of life on the Health Assessment Questionnaire.
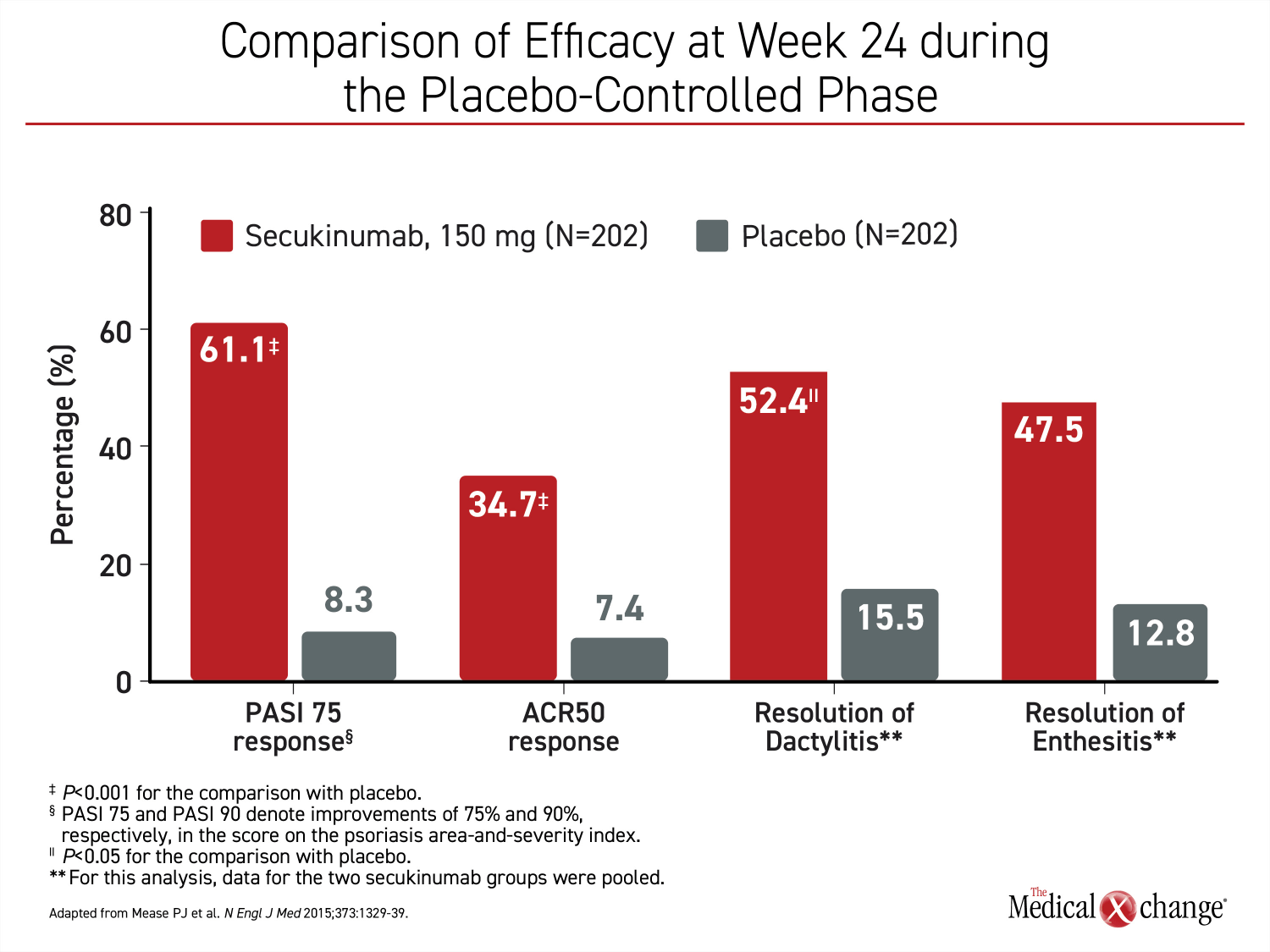
The first IL-17i, secukinumab was approved for PsA in 2015 on the basis of the phase 3 FUTURE 1 trial.17 The primary endpoint in that trial was a sustained improvement in joint involvement as measured with improvement on ACR criteria. Additional outcomes supported the efficacy of secukinumab on multiple PsA outcomes (Figure 4). Subsequent studies also supported benefit from this biologic on skin lesions,18 enthesitis,19 and dactylitis.20 In a recently-published direct multicenter comparison to adalimumab in patients with PsA, secukinumab provided a numerically superior improvement in joint symptoms measured with ACR20 (76.4% vs. 68.3%; P=0.175) and a statistically significant complete clearance of skin lesions measured by PASI 100 (39.1% vs. 23.8%; P=0.013).21
IL-17 is the major effector cytokine in the pathogenesis of both psoriasis and PsA, according to a recent review of this target.22 IL-17A, to which secukinumab binds, is produced by a wide variety of immune cells, including T cells and mast cells. When upregulated, it affects keratinocytes, neutrophils, osteoclasts, chondrocytes, and endothelial cells. While secukinumab has now been joined by other biologics acting on this pathway, the inhibitor of IL-17A has been credited with large improvements across all of the domains in which PsA is expressed.22
The molecular pathophysiology of PsA, like other autoimmune diseases, is a complex product of innate immune cell dysregulation and dysregulation of cytokine expression involving multiple pathways.23 Clinical studies have proven that disease expression and progression can be modified by inhibiting several of these pathways, but the expanding number of biologic and targeted therapies for moderate-to-severe disease should not confuse clinical choices. With uncertainty about which pathways dominate in disease expression across different domains of PsA, treatment choices require empirical data. This not only involves evidence of sustained efficacy, but a demonstration of sustained safety, which is particularly important for therapies that target immunological pathways.
For example, drugs in the JAKi class represent the first oral targeted therapies approved in PsA. This might be perceived as a relative advantage by patients even though daily dose requirements raise concern about long-term compliance. Empirically, there is no compelling evidence that drugs in the JAKi class improve long-term outcomes, but there is concern that they may induce long-term risks, including viral reactivations, lymphoma, and venous thromboembolism.24 Similarly, although the selective IL-23i guselkumab has been found reasonably well tolerated in short-term studies,25 its efficacy has not been established across all PsA domains, and its long-term safety remains unknown.
In the new GRAPPA treatment recommendations, some of the variability in the specific recommendations for moderate-to-severe disease across the six domains stems from disparities in available evidence. However, the strong recommendation conferred to TNFi and IL-17i across all six PsA domains was not restricted to hard endpoints regarding safety and efficacy as derived from controlled trials. Rather, preferred treatments were also selected on the basis of a neutral or minimal adverse effect on comorbidities and a favourable effect on outcomes dependent on patient experience, such as improvements in quality of life. This is reflected by the endorsement of patient participants in the GRAPPA panel.
Summary
The GRAPPA treatment recommendations now being prepared for publication are an update from those published in 2016. There are no substantial changes in the list of treatments for mild disease across the six PsA domains of disease activity. However, the recommendations for moderate-to-severe disease have been revised. In the new recommendations, biologics, targeted therapies, or both, are now recommended as first-line therapies in moderate-to-severe disease for all six PsA domains. Of biologics and targeted therapies, only TNF inhibitors and IL-17 inhibitors have been identified as preferred for each of the six domains.
IL-12/23 inhibitors, such as ustekinumab, or the selective IL-23 inhibitor guselkumab, which have been added to therapeutic options in PsA since 2016, are not recommended for use against the axial domain of PsA in the new GRAPPA treatment recommendations. This distinction is not only relevant for those with prominent axial symptoms, but to a larger GRAPPA principle of treatment selection, which calls for preference to therapies that address as many domains as possible. Relative to TNFi and IL-17i, the availability of additional biologics and targeted therapies are important for patients with symptoms not adequately controlled on these first-line agents.
References
1. Ritchlin CT, Colbert RA, Gladman DD. Psoriatic Arthritis. N Engl J Med 2017;376(10):957-970. DOI: 10.1056/NEJMra1505557.
2. Taylor W, Gladman D, Helliwell P, et al. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 2006;54(8):2665-73. DOI: 10.1002/art.21972.
3. Eder L, Widdifield J, Rosen CF, et al. Trends in the Prevalence and Incidence of Psoriasis and Psoriatic Arthritis in Ontario, Canada: A Population-Based Study. Arthritis Care Res (Hoboken) 2019;71(8):1084-1091. DOI: 10.1002/acr.23743.
4. FitzGerald O, Ogdie A, Chandran V, et al. Psoriatic arthritis. Nat Rev Dis Primers 2021;7(1):59. DOI: 10.1038/s41572-021-00293-y.
5. Coates LC, Helliwell PS. Psoriatic arthritis: state of the art review. Clin Med (Lond) 2017;17(1):65-70. DOI: 10.7861/clinmedicine.17-1-65.
6. Coates LC, Soriano E, Corp N, H. B. The Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) treatment recommendations 2021. European League Against Rheumatism (EULAR) 2021;2021 Virtual Meeting.
7. Coates LC, Kavanaugh A, Mease PJ, et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis 2015 Treatment Recommendations for Psoriatic Arthritis. Arthritis Rheumatol 2016;68(5):1060-71. DOI: 10.1002/art.39573.
8. Gladman D, Rigby W, Azevedo VF, et al. Tofacitinib for Psoriatic Arthritis in Patients with an Inadequate Response to TNF Inhibitors. N Engl J Med 2017;377(16):1525-1536. DOI: 10.1056/NEJMoa1615977.
9. Papp K, Gordon K, Thaci D, et al. Phase 2 Trial of Selective Tyrosine Kinase 2 Inhibition in Psoriasis. N Engl J Med 2018;379(14):1313-1321. DOI: 10.1056/NEJMoa1806382.
10. McInnes IB, Anderson JK, Magrey M, et al. Trial of Upadacitinib and Adalimumab for Psoriatic Arthritis. N Engl J Med 2021;384(13):1227-1239. DOI: 10.1056/NEJMoa2022516.
11. Deodhar A, Helliwell PS, Boehncke WH, et al. Guselkumab in patients with active psoriatic arthritis who were biologic-naive or had previously received TNFalpha inhibitor treatment (DISCOVER-1): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet 2020;395(10230):1115-1125. DOI: 10.1016/S0140-6736(20)30265-8.
12. Gossec L, Baraliakos X, Kerschbaumer A, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis 2020;79(6):700-712. DOI: 10.1136/annrheumdis-2020-217159.
13. Shah K, Paris M, Mellars L, Changolkar A, Mease PJ. Real-world burden of comorbidities in US patients with psoriatic arthritis. RMD Open 2017;3(2):e000588. DOI: 10.1136/rmdopen-2017-000588.
14. Mantravadi S, Ogdie A, Kraft WK. Tumor necrosis factor inhibitors in psoriatic arthritis. Expert Rev Clin Pharmacol 2017;10(8):899-910. DOI: 10.1080/17512433.2017.1329009.
15. Gladman DD. Adalimumab, etanercept and infliximab are equally effective treatments for patients with psoriatic arthritis. Nat Clin Pract Rheumatol 2008;4(10):510-1. DOI: 10.1038/ncprheum0880.
16. Atteno M, Peluso R, Costa L, et al. Comparison of effectiveness and safety of infliximab, etanercept, and adalimumab in psoriatic arthritis patients who experienced an inadequate response to previous disease-modifying antirheumatic drugs. Clin Rheumatol 2010;29(4):399-403. DOI: 10.1007/s10067-009-1340-7.
17. Mease PJ, McInnes IB, Kirkham B, et al. Secukinumab Inhibition of Interleukin-17A in Patients with Psoriatic Arthritis. N Engl J Med 2015;373(14):1329-39. DOI: 10.1056/NEJMoa1412679.
18. McInnes IB, Mease PJ, Ritchlin CT, et al. Secukinumab sustains improvement in signs and symptoms of psoriatic arthritis: 2 year results from the phase 3 FUTURE 2 study. Rheumatology (Oxford) 2017;56(11):1993-2003. DOI: 10.1093/rheumatology/kex301.
19. Coates LC, Wallman JK, McGonagle D, et al. Secukinumab efficacy on resolution of enthesitis in psoriatic arthritis: pooled analysis of two phase 3 studies. Arthritis Res Ther 2019;21(1):266. DOI: 10.1186/s13075-019-2055-z.
20. Garcia-Montoya L, Marzo-Ortega H. The role of secukinumab in the treatment of psoriatic arthritis and ankylosing spondylitis. Ther Adv Musculoskelet Dis 2018;10(9):169-180. DOI: 10.1177/1759720X18787766.
21. Gottlieb AB, Merola JF, Reich K, Behrens F. Efficacy of secukinumab and adalimumab in patients with psoriatic arthritis and concomitant moderate-to-severe plaque psoriasis: results from EXCEED, a randomized double-blind head-to-head monotherapy study. Br J Dermatol 2021;April 29.
22. Blauvelt A, Chiricozzi A. The Immunologic Role of IL-17 in Psoriasis and Psoriatic Arthritis Pathogenesis. Clin Rev Allergy Immunol 2018;55(3):379-390. DOI: 10.1007/s12016-018-8702-3.
23. Carvalho AL, Hedrich CM. The Molecular Pathophysiology of Psoriatic Arthritis-The Complex Interplay Between Genetic Predisposition, Epigenetics Factors, and the Microbiome. Front Mol Biosci 2021;8:662047. DOI: 10.3389/fmolb.2021.662047.
24. Cohen SB, Tanaka Y, Mariette X, et al. Long-term safety of tofacitinib up to 9.5 years: a comprehensive integrated analysis of the rheumatoid arthritis clinical development programme. RMD Open 2020;6(3). DOI: 10.1136/rmdopen-2020-001395.
25. McHugh J. IL-23 inhibitor guselkumab shows promise for PsA. Nat Rev Rheumatol 2020;16(5):247. DOI: 10.1038/s41584-020-0420-6.