Psychiatry
CANMAT Guidelines: Expert Review and Commentary
Primary Care: Updated CANMAT Guidelines for Navigating Poor Response in Major Depressive Disorder
Sidney Kennedy, MD, FRCPC
Professor and Arthur Sommer-Rotenberg Chair, University of Toronto
Scientist, Li Ka Shing Knowledge Institute and Krembil Research Institute
Director, Homewood Research Institute
Toronto, Ontario
Valérie Tourjman, MD, FRCPC
Associate Professor, Université de Montréal
Medical Director of electroconvulsive therapy services, Institut Universitaire en Santé Mentale de Montréal (IUSMM)
Medical Coordinator for third-line psychiatric treatment services, IUSMM
Montreal, Quebec
Since the last full set of Canadian Network for Mood and Anxiety Treatments (CANMAT) guidelines for managing major depressive disorder (MDD) were published in 2016,1 the approach to an inadequate response to first-line pharmacologic therapy has been revised.2 The evidence-based recommendations, which are suitable for both specialists and non-specialists, now highlight a shift to earlier introduction of adjunctive pharmacologic therapy in patients with partial response following dose optimization of an initial antidepressant. This is now preferred over serial switching of monotherapies. The new guidelines recognize the substantial rates of failure on first-line MDD therapies and the progressively diminishing response to alternative drugs. Overall, about half of patients fail a first-line treatment. The new guidelines emphasize collaboration between patient and treater provider and provide logical next steps for specialists and primary care physicians to navigate when referrals are impractical or unavailable.
Therapeutic Approach in Primary Care
According to data cited in the newly released CANMAT guidelines, which were published in 2024 and based on a data review completed in 2023, the proportion of individuals with MDD in Canada who are diagnosed and receive adequate treatment might be as few as 20%. Characterized as a major public health problem,3 depression is disabling, adversely affects quality of life, impairs productivity, and is potentially life threatening for those who act on suicide ideation.4
For an exclusive discussion with Dr. Sidney Kennedy and Dr. Jeffrey Habert on the impact to clinical practice, click here
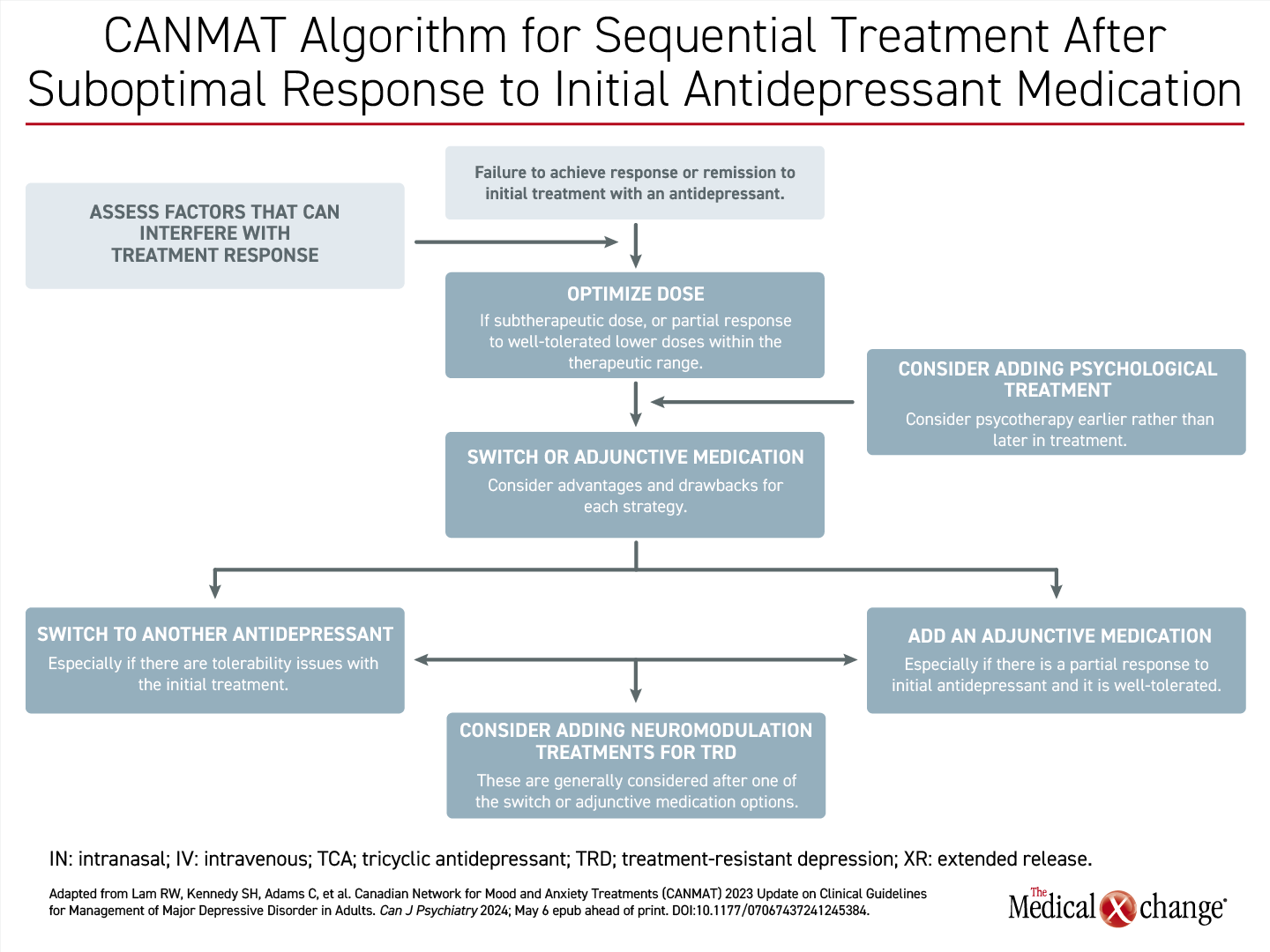
To navigate treatment, the CANMAT guidelines emphasize the importance of a therapeutic alliance with the patient, providing a basis for monitoring response, safety, and tolerability. Due to the frequency with which therapy must be switched or adjusted in the event of an inadequate response or avoid side effects, clinicians should be prepared in follow-up to make modifications, which include moderating the dose, switching to an alternative drug, or adding an adjunctive agent.
For the goal of offering an adequately tolerated therapy to restore functionality and quality of life to the premorbid level, the addition of an adjunctive therapy as an alternative to switching between drugs for a partial or inadequate response has been moved up in the algorithm. According to the new CANMAT guidelines, only about half of patients achieve an adequate response, defined as at least a 50% reduction in symptom severity, on antidepressant monotherapy offered in the first-line.
What Follows First-line Antidepressant Failure?
For the estimated 50% of patients who do not achieve an adequate response to a first- or second-line therapy, the new guidelines prefer the term difficult-to-treat depression over
treatment-resistant depression, a term once used commonly to define an inadequate response to 2 or more antidepressants. The broader term focuses attention on patient-prioritized outcomes and recognizes that therapies often yield incremental gains even if full remission is not achieved. This broader term also eliminates any implied blame on the patient.
In this context, the guidelines recommend a collaborative approach between clinician and patient to evaluate treatment with patient-rated outcome measures (PROMs) along with traditional clinician administered scales for monitoring response. For PROMs, which encourage patients to participate in assessing their own treatment, digital health technologies (DHIs) may be helpful. DHIs incorporate mobile apps and internet programs to track symptoms over time, capturing longitudinal changes in symptoms.
Although DHIs, which vary substantially in quality, are not interchangeable, and should be vetted for their reliability, monitoring PROMs with DHIs or other methods is useful for tracking the impact of pharmacologic therapies, lifestyle changes, and such techniques as mindfulness or meditation to improve mood. In order to circumvent the pejorative and discouraging characterization of an incomplete or partial response as “resistance,” the emphasis on the PROMs strategy allows therapeutic gains with multiple strategies to be employed concomitantly to reach adequate symptom control.
A Shift Towards Earlier Use of Adjunctive Medication
This orientation of building on gains in symptom control is consistent with the concept of augmenting first-line therapies with adjunctive pharmacologic therapies rather than cycling through a series of monotherapies that by themselves have a low likelihood of providing a remission. As in the previous guidelines, it remains appropriate to verify adherence and adequate dosing of the first-line therapy prior to embarking on an alternative therapy, but a partial response might not justify abandoning a first- or second-line therapy for another.
The shift in the guidelines recognizes the evidence that symptom control diminishes with serial single agents whether from the same or different pharmacologic class. The guidelines, which based this recommendation like others on systematic reviews and meta-analyses, do not rule out benefit from switching, particularly if there was no or minimal benefit from the initial treatment. Yet, several advantages are listed for adding a second medication rather than switching. These include adding a second mechanism of action and the potential for minimizing adverse events with low doses of two drugs rather than higher doses of a single drug.
In a revised treatment algorithm, the decision to switch therapy or provide an additional adjunctive antidepressant is recommended within weeks after patients report an inadequate response on a first or second antidepressant therapy administered in an optimized dose (Figure 1). According to the guidelines, if there is not at least a 20% reduction symptom scores by 4 weeks, the likelihood of a satisfactory response at 8 or 12 weeks is low. Switching to an alternative single agent is sensible when failure of the initial agent is due to intolerance, but going directly to adjunctive therapy is attractive when there is a partial although inadequate response to the first-line therapy.
First-line Adjunctive Therapies: Benefits of Serotonin-dopamine Modulators
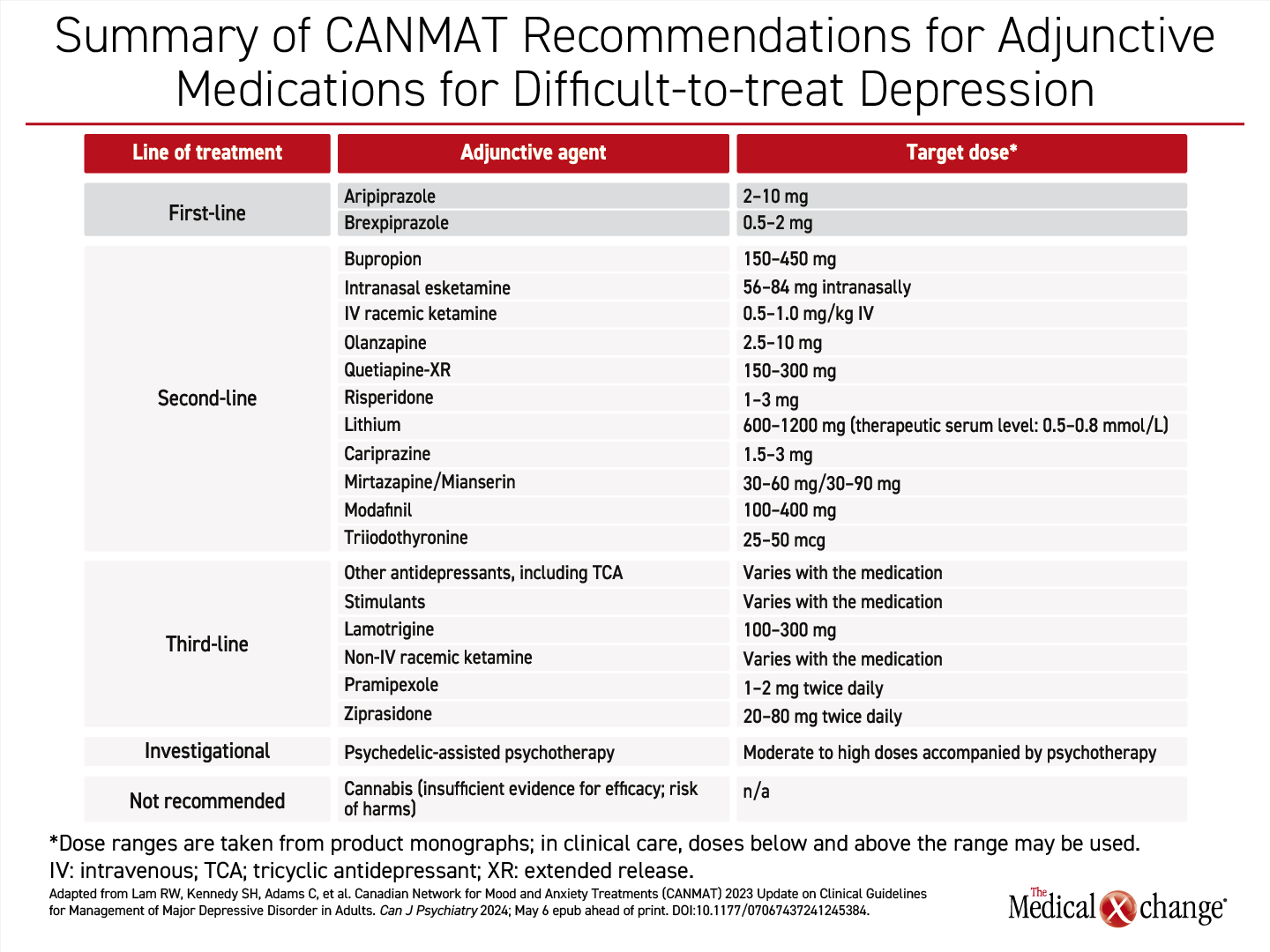
The preferred adjunctive agents in the CANMAT guidelines are specified as brexpiprazole or aripiprazole (Table 1). Both are licensed for this indication in Canada on the basis of controlled trials.
Brexpiprazole, which was initially approved for schizophrenia, has multiple psychiatric indications, of which the most recent was granted for control of agitation associated with Alzheimer’s dementia. Approved in Canada as an adjunctive therapy for MDD since 2019, this serotonin-dopamine modulator has shown demonstrated efficacy in multiple placebo-controlled trials that were summarized in a 2019 meta-analysis.5 Of the 9 trials included, all evaluated brexpiprazole as an adjunctive treatment in MDD patients who had failed at least 1 prior antidepressant therapy. These trials support the efficacy and general tolerability of brexpiprazole.
Aripiprazole, also a serotonin-dopamine modulator initially licensed for schizophrenia, was approved in Canada as an adjunctive treatment for MDD approximately 5 years earlier than brexpiprazole. In a meta-analysis of eight randomized and controlled studies of this agent for adjunctive use in MDD, in comparison to placebo or an active control, aripiprazole was also effective on objective measures of response. Side effects were generally more common on aripiprazole relative to comparator arms.6
Brexpiprazole and aripiprazole have never been compared directly in a clinical trial, but they differ pharmacologically. Brexpiprazole has more potent activity on serotonin receptors 5-HT2A and 5-HT1A but less intrinsic activity on the dopamine receptor D2.7 Brexpiprazole also has less binding on the histamine H1 receptor. The reduced activity on the D2 receptor predicts a lower risk of akathisia and extrapyramidal symptoms.8
These two serotonin-dopamine modulators are preferred first-line adjunctive therapies for MDD in the CANMAT guidelines on the basis of the consistency of the evidence of benefit and the lower relative risk of adverse events than the second-line agents, many of which were also initially developed for schizophrenia and now have multiple indications. Used in low relative doses as adjunctive treatments for MDD, the lower risk of adverse events with serotonin-dopamine modulators relative to older atypical antipsychotics is further mitigated.
Second-line adjunctive therapies listed in the updated CANMAT guidelines include bupropion, in addition to other atypical antipsychotics, such as olanzapine and risperidone, but the side effects of serotonin-dopamine modulators are generally lower than the older agents with an antipsychotic indication when used in the doses commonly employed in adjunctive treatment.
In the event of an inadequate response to a first-line antidepressant plus an adjunctive therapy, treatment options include neuromodulation or more intensive combination of therapies that might better be administered by specialists, but the guidelines suggest that management of MDD can be achieved in the primary care setting with the stepwise algorithm outlined.
Summary
MDD is a highly prevalent disorder in Canada, is underdiagnosed and is often inadequately treated despite an array of effective treatments. With first-line antidepressants, about half of patients achieve remission, emphasizing the importance of monitoring response and considering alternative approaches when symptom control is inadequate.
In these newly released CANMAT guidelines, the emphasis placed on adjunctive treatments if a first or second antidepressant is not adequately effective represents a major change. As opposed to serial switches among monotherapies, adjunctive therapy offers a greater opportunity for adequate symptom control while preserving partial response achieved on initial treatment. A collaborative approach with the patient can help define an inadequate response. The guidelines outline a logical sequence of steps for the primary care physician to move through a treatment algorithm before resorting to a specialist referral.
References
- Patten SB, Williams JV, Lavorato DH, Wang JL, McDonald K, Bulloch AG. Major Depression in Canada: What Has Changed Over the Past 10 Years? Can J Psychiatry 2016;61(2):80-5. DOI: 10.1177/0706743715625940.
- Lam RW, Kennedy SH, Adams C, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2023 Update on Clinical Guidelines for Management of Major Depressive Disorder in Adults. Can J Psychiatry 2024; May 6 epub ahead of print. DOI:10.1177/07067437241245384.
- McLaughlin KA. The public health impact of major depression: a call for interdisciplinary prevention efforts. Prev Sci 2011;12(4):361-71. DOI: 10.1007/s11121-011-0231-8.
- Jain S, Gupta S, Li VW, Suthoff E, Arnaud A. Humanistic and economic burden associated with depression in the United States: a cross-sectional survey analysis. BMC Psychiatry 2022;22(1):542. DOI: 10.1186/s12888-022-04165-x.
- Kishi T, Sakuma K, Nomura I, Matsuda Y, Mishima K, Iwata N. Brexpiprazole as Adjunctive Treatment for Major Depressive Disorder Following Treatment Failure With at Least One Antidepressant in the Current Episode: a Systematic Review and Meta-Analysis. Int J Neuropsychopharmacol 2019;22(11):698-709. DOI: 10.1093/ijnp/pyz040.
- Luan S, Wan H, Zhang L, Zhao H. Efficacy, acceptability, and safety of adjunctive aripiprazole in treatment-resistant depression: a meta-analysis of randomized controlled trials. Neuropsychiatr Dis Treat 2018;14:467-477. DOI: 10.2147/NDT.S156619.
- Stahl SM. Mechanism of action of brexpiprazole: comparison with aripiprazole. CNS Spectr 2016;21(1):1-6. DOI: 10.1017/S1092852915000954.
- Citrome L. The ABC’s of dopamine receptor partial agonists – aripiprazole, brexpiprazole and cariprazine: the 15-min challenge to sort these agents out. Int J Clin Pract 2015;69(11):1211-20. DOI: 10.1111/ijcp.12752.