Cardiology
10th European Stroke Organization Conference (ESOC) 2024
Stroke Therapy Targeted at Platelet Glycoprotein Still Considered Promising Despite Negative Trial
Basel – For acute stroke, a novel monoclonal antibody targeting platelet glycoprotein VI (GPVI) failed to meet its efficacy endpoint in a phase 2/3 trial. The results were disappointing, but a post hoc analysis suggests that a positive result from a novel anticlotting agent was masked by imbalances at baseline in the study arms. Furthermore, the results were inconsistent with a previous early phase clinical study and with experimental studies conducted during its development. Importantly, there was no association with the novel therapy and an increase in major bleeding, which is the key potential advantage of inhibiting the GPVI target.
Based on these factors, the negative result of this trial, called ACTISAVE, will not affect a series of planned or ongoing controlled trials with the novel agent, glenzocimab, in acute stroke, myocardial infarction, and other thrombotic disorders for which it is still considered to hold promise, according to Dr. Martin Köhrmann, MD, Deputy Hospital Director, Neurological University Hospital, Essen, Germany.
Antithrombotic Might Be Free of Bleeding Risk
The unique feature of the GPVI target, which interacts with collagen, fibrinogen, fibrin, and fibronectin to promote platelet activation and clot formation, is that inhibition appears to have little or no effect on hemostasis. For anticlotting drugs, an agent that separates its antithrombotic effect from risk of bleeding has been a major unmet need and could, if shown effective, be a major advantage by addressing the Achille’s heel of other anticlotting therapies.
For anticlotting drugs, an agent that separates its antithrombotic effect from risk of bleeding has been a major unmet need.
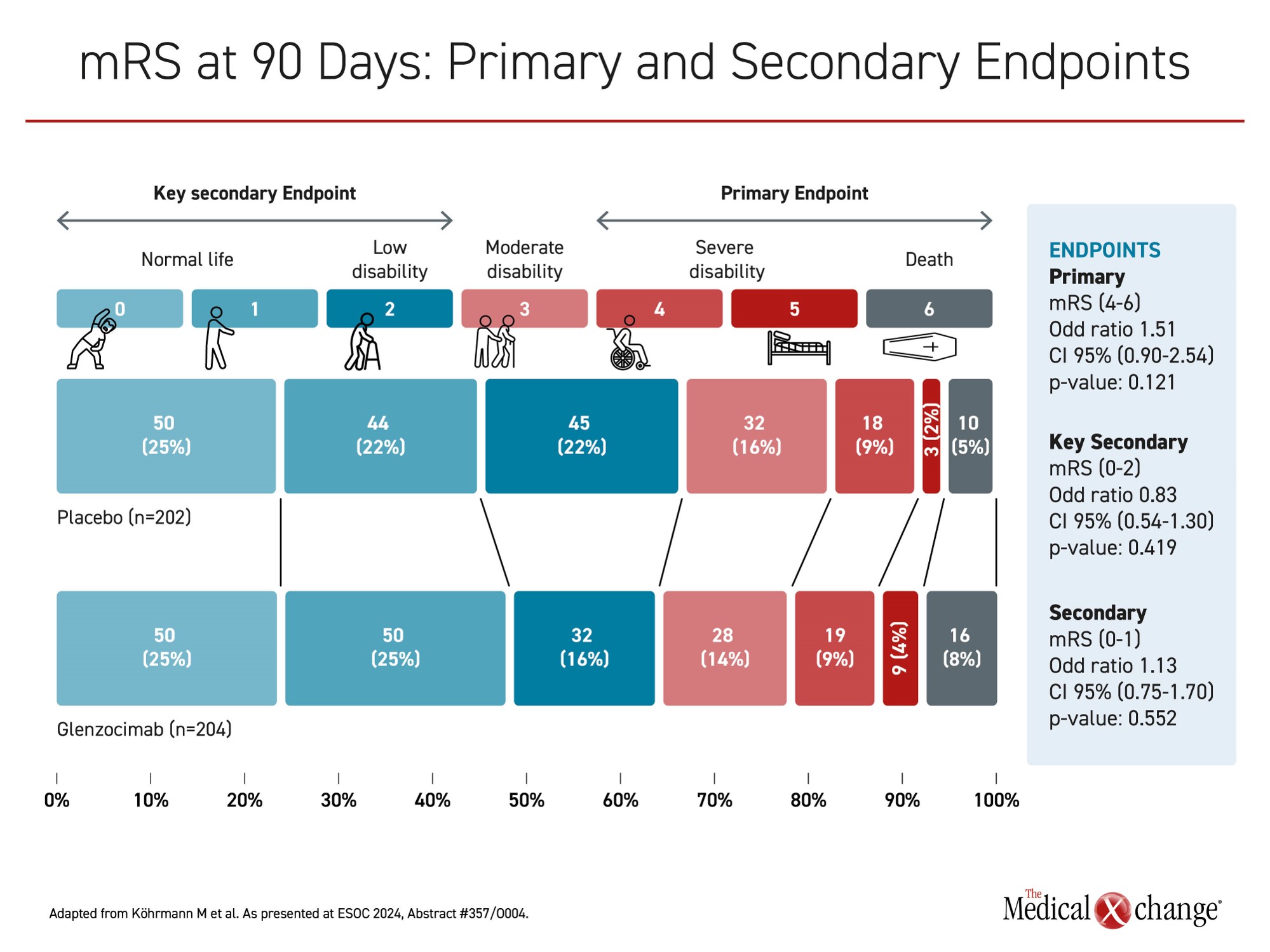
In the ACTISAVE trial, 438 acute stroke patients were randomized across approximately 60 centers in 10 countries to intravenous infusion of 1000 mg of glenzocimab or placebo within 4.5 hours of symptom onset. The primary efficacy endpoint was a modified Rankin Scale (mRS) score (4-6), which means severe disability or death at day 90. Bleeding events, particularly intracranial hemorrhage (ICH), was a key safety endpoint.
When placebo and glenzocimab patients were compared at 90 days, the proportion with a mRS score of 4 or higher was numerically but not significantly greater in the active treatment arm (35% vs. 32%; P=0.121). For other outcomes, such as patients with mRS score of ≤2, there were some numerical advantages in favour of glenzocimab, but, no difference in mRS score, whether favourable or unfavourable to the study agent, reached significance (Figure 1).
Bleeding Events Were Similar to Placebo
The overall rate of bleeding events on glenzocimab relative to placebo was essentially the same (18% vs. 19%). They included the rates of both non-symptomatic (19% vs. 20%) and symptomatic ICH (4% vs. 3%).
Based on earlier experimental and clinical studies with glenzocimab, including a recently published phase 1b/2a trial called ACTIMIS, the neutral efficacy outcome was unexpected. The primary outcome of the early phase dose-finding ACTIMS study, which randomized patients to one of four doses of glenzocimab or placebo within 4.5 hours of a stroke, involved safety. At the highest dose of 1000 mg, which was the dose employed in ACTISAVE, the experimental therapy was associated with a lower risk of all-cause death (7% vs. 21%). Moreover, the highest dose of glenzocimab relative to placebo was associated with lower rates of non-symptomatic hemorrhage (31% vs. 50%) and ICH (0% vs. 10%).
[In the prior ACTIMIS study] the experimental therapy was associated with a lower risk of all-cause death
In a post hoc ACTISAVE analysis conducted to explore potential reasons for the neutral result, several substantial imbalances between active treatment and placebo groups at baseline were identified. Of those identified three were considered potentially relevant. One was a nearly two times higher rate of diabetes in the experimental arm (23% vs. 12%). Another was a higher baseline NIH Stroke Scale score (17 vs. 14). Finally, the lower expanded treatment in cerebral infarction (eTICI) scores in the experimental arm (61% vs. 78%) narrowed an opportunity to show a therapeutic advantage for the experimental arm.
Benefit of Glenzocimab Seen in Subset
When patients with an eTICI score of 3 were compared, “the proportion of patients with a successful recanalization in the glenzocimab arm was more favourable,” Dr. Köhrmann reported. In this subset of 34 glenzocimab and 37 placebo patients, the proportion of those achieving mRS of ≤1, meaning no significant disability, was nearly double in favour of the active therapy (58% vs. 32%) (Figure 2).
[In a select subgroup] the proportion of those achieving mRS of ≤1 […] was nearly double in favour of the active therapy.
This post hoc analysis can only be hypothesis generating, but Dr. Köhrmann said they will not affect the conduct of the phase 2/3 GREEN or GALICE trials. In GREEN, which is already enrolling patients, glenzocimab is being evaluated as an adjunct to mechanical thrombectomy in patients with acute ischemic stroke. Patients are being randomized within 24 hours of symptom onset to thrombectomy alone or thrombectomy plus glenzocimab. The primary endpoint is functional outcome as measured with mRS at 90 days. In GALICE, which has not yet started, glenzocimab is also being evaluated as an adjunct to mechanical thrombectomy, but the study will be limited to those with a large stroke and proximal occlusion eligible for thrombectomy.
Ultimately, the premise that GPVI has the potential to inhibit clot formation without increasing the risk of bleeding in stroke remains viable, according to Dr. Köhrmann, who explained that this is the hypothesis driving the GREEN and GALICE studies. A separate randomized, placebo-controlled trial called LIBERATE, which has also already begun enrollment, will test the ability of the same 1000 mg dose of glenzocimab to safely reduce infarct size when offered to ST-elevated myocardial infarction (STEMI) patients undergoing a percutaneous intervention.
“The ACTISAVE study did not meet its primary endpoint, and there was no evidence of a significant benefit, but coming trials might still show a role for this agent,” Dr. Köhrmann said.
Conclusion
The novel monoclonal antibody glenzocimab targets GPVI and is associated with platelet activation but does not appear to have any unwanted effect on hemostasis. These features would be expected to have a greater benefit-to-risk ratio than other anticlotting therapies used in stroke and thrombotic diseases. Although the phase 2/3 ACTISAVE trial did not associate glenzocimab with protection from disability 90 days after an acute stroke, the results are being questioned due to imbalances expected to disadvantage the experimental arm. This imbalance coupled with earlier evidence of benefit means the negative result from ACTISAVE will not preclude further development of glenzocimab.