HIV
18th Conference on Retroviruses and Opportunistic Infections (CROI)
Mortality in Patients with HIV: The Shift to Age-Related Diseases
Boston – Between 2005 and 2009, when the most recent analysis was conducted, only 15% of deaths in a human immunodeficiency virus (HIV) cohort could be directly attributed to immune dysfunction. The remaining 85% of deaths were due to other causes including heart disease, liver disease, and non-acquired immunodeficiency syndrome (AIDS) malignancies. These figures emphasize the importance of a major reorientation in HIV care in which new emphasis has been placed on identifying which antiretroviral agents pose the least risk for exacerbating disease processes in major end organs. Based on data presented at the 18thCROI, this information continues to evolve in important ways. New data has been presented on antiretrovirals in relation to kidney function, cardiovascular risk, and dementia. Separating the effects of HIV from the effects of the therapies is often challenging, but it is now clear that antiretroviral agents differ for the specific risks they pose in regard to age-related diseases. This encourages treatment strategies that consider long-term health on top of HIV suppression.
Major Challenges in HIV Care
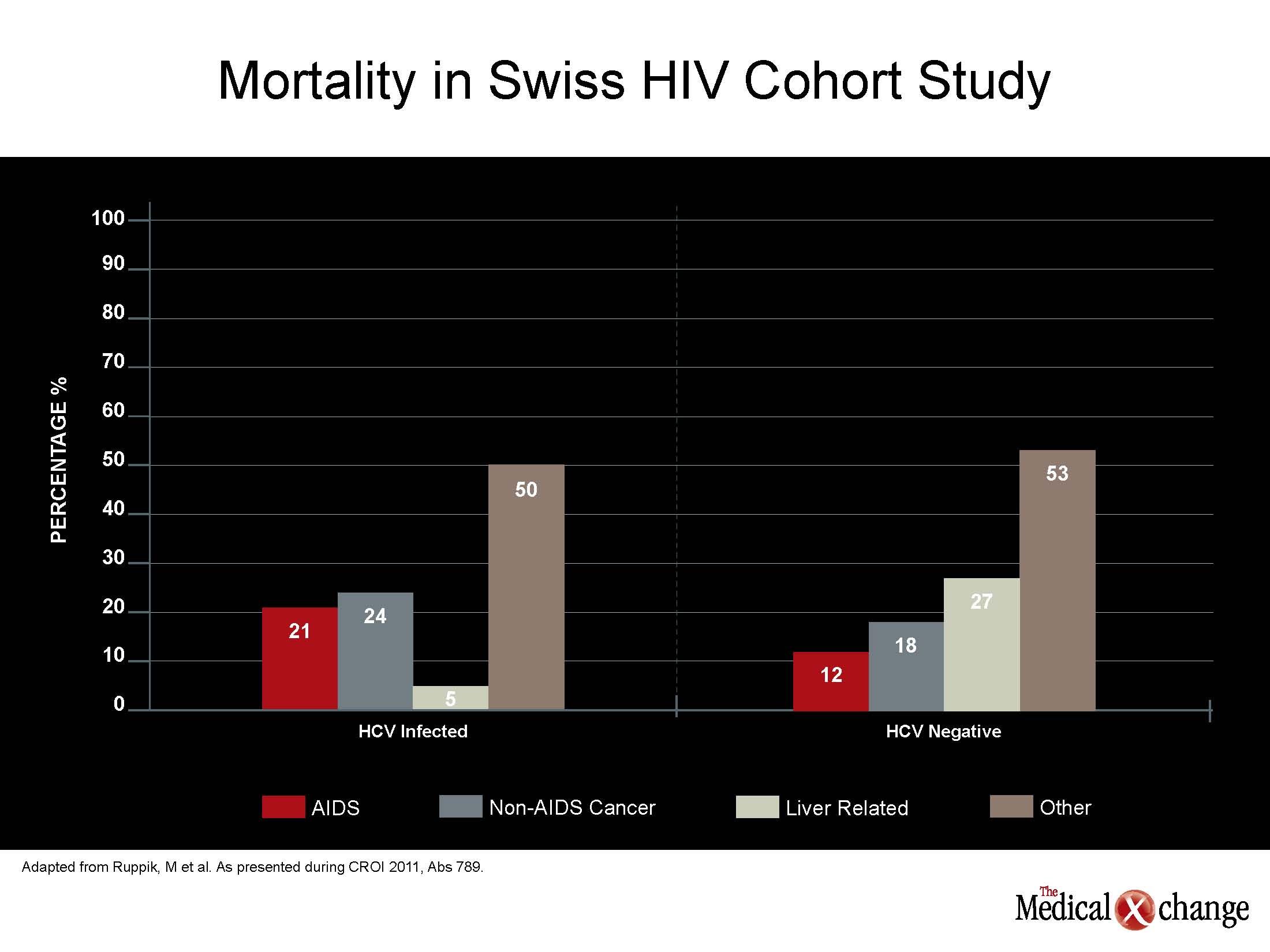
New data from several large cohorts confirm that protection against age-related pathophysiologic processes, rather than a failing immune system, is the major challenge in human immunodeficiency virus (HIV) care. In the Swiss HIV Cohort Study (SHCS), only 15% of deaths were attributed to acquired immunodeficiency syndrome (AIDS). In mortality data generated by 15,920 HIV patients enrolled in EuroSIDA, the risk of a non-AIDS-related death, which represented the majority of deaths, increased with cumulative exposure to combination antiretroviral therapy (cART). In a North American study, estimated glomerular filtration rate (eGFR), hepatitis C virus (HCV) serostatus, age and hemoglobin were found to provide better mortality prediction than HIV-related factors alone (see Figure 1) (Fig. 1).
“Non-HIV co-morbidities, particularly diabetes mellitus, cardiovascular disease, non-AIDS defining malignancies and osteoporosis, are becoming critical in HIV care….”
“Non-HIV co-morbidities, particularly diabetes mellitus,cardiovascular [CV] disease, non-AIDS defining malignancies and osteoporosis, are becoming critical in HIV care. These are frequently age-related, reflecting the changing needs of patients who achieve long-term survival on effective drug regimens,” reported Dr. Barbara Hasse, Division of Infectious Diseases and Hospital Epidemiology, University Hospital, Zurich, Switzerland. One of the lead investigators in the SHCS, Dr. Hasse reported that these data, which include more than 9,000 patients, emphasize the importance of non-AIDS pathology in numerous ways. For example, the median CD4 count at the time of death in this SHCS analysis was 321 cells/mm3, which represents only modest immunodeficiency.
Renal Impairment and HIV Outcomes
“Renal function at baseline is an independent predictor of mortality in HIV-infected patients and should be routinely assessed”
“Renal function at baseline is an independent predictor of mortality in HIV-infected patients and should be routinely assessed,” reported the author of this study, Ms. Fowzia Ibrahim, Research Fellow in Medical Statistics, King’s College, London, UK. Deriving the data from 20,132 HIV patients in the UK Consumer Health Information Consortium, of which 9.4% died over a median 5.7 years of follow-up, Ms. Ibrahim indicated that renal impairment, which is common in aging individuals, appears to be accelerated in patients with HIV, and greater appreciation for this phenomenon is needed. Close monitoring of renal function in aging individuals with HIV infection appears to be warranted, but there is increasing debate about whether strategies to reduce risk should be implemented in high risk individuals early in the course of treatment. For example, a new study compared the effect of tenofovir, a commonly used antiretroviral agent that is closely associated with renal dysfunction, in patients co-infected with hepatitis B virus (HBV). In these, a baseline fibrosis level greater than 3.0 had a 3.74 increased relative risk (P=0.005) of renal impairment 24 months after starting tenofovir than those with less fibrosis after adjusting for age, race, viral load, and other factors. The average age in this population of 137 patients was only 41.7 years. The results emphasize that although “renal impairment is a feared side effect of tenofovir… risk is not evenly distributed,” reported Dr. Karine Lacombe, PhD, Hospital St. Antoine, INSERM, Paris, France. While patients with liver disease due to hepatitis infection may be at particular risk of renal impairment from this nucleoside reverse transcriptase inhibitor (NRTI), the degree of liver pathology appears to be important for determining the magnitude of risk. The point that baseline risk strongly influences the potential for renal impairment from antiretroviral therapy was reinforced by another study of 890 patients. In this study, 573 (64.4%) had normal creatinine clearance, defined as eGFR ≥ 90 mL/min/1.73 m2, at baseline. Of the remaining, 30.4% had mild impairment (60 to 80 mL/min/1.73 m2) and 5.2% had moderate impairment (30 to 59 mL/min/1.73 m2). All patients received tenofovir. Over follow-up of up to five years, the rates of nephrotoxicity were very low in those with normal (0.4 per 100 person-years) and mild baseline renal impairment (2.5 per 100 person-years), but climbed to 10.7 per 100-person years in those with moderate impairment. The increasing rate of renal impairment with declining eGFR was paralleled by the hazard ratio (HR) for all-cause mortality. By the end of follow-up, the HR for mortality was about five times greater in those with moderate renal impairment compared to those with no or mild renal impairment. According to the lead author of this study, Dr. Alana Brennan, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa, “much of the incident renal dysfunction in tenofovir-treated patients is likely related to pre-existing renal pathology.” From a practical perspective, Dr. Brennan suggested that “with expanded use of tenofovir, screening for renal insufficiency prior to treatment initiation is essential to reduce nephrotoxicity.”
CV Disease: Making Sense of Risk Factors
In aging HIV patients, the greatest cause of premature death is expected to be CV disease, but the risk factors appear to be multifactorial and complex. In new findings drawn from the U.S. Nationwide Inpatient Sample (NIS) database, which has data on 293,175 patients who presented with an acute coronary syndrome in the year 2008, the median age of HIV-positive patients was 50, which was five years younger (P<0.01) than in individuals who were HIV-negative. The OR of renal disease was 3.03 (P<0.05) greater in the HIV-positive patients, and patients with HIV were more likely to develop acute renal failure during admission (14.9% vs. 8.2%; P<0.01). However, patients with HIV were less likely to have hypertension (55.8% vs. 61%; P<0.01), hyperlipidemia (9.2% vs. 12.5%; P<0.01), or diabetes mellitus (16.1% vs. 26.2%; P<0.01). In-hospital mortality was higher in those with HIV (3.3% vs. 2.3%; P<0.01).
“According to our data, HIV-positive patients admitted to a hospital for acute coronary syndrome are younger, have fewer traditional CV risk factors but have a higher mortality rate.”
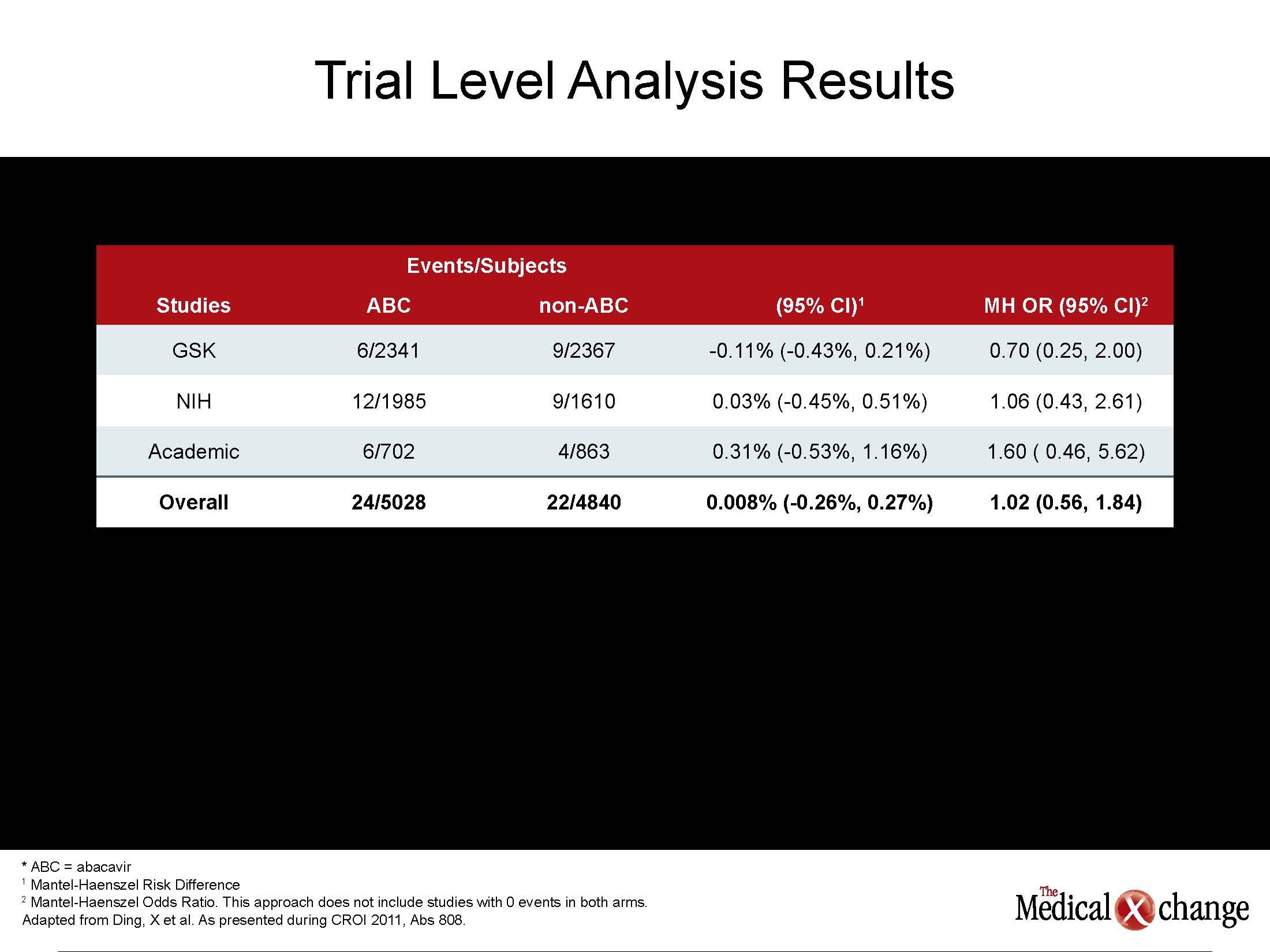
“According to our data, HIV-positive patients admitted to a hospital for acute coronary syndrome are younger, have fewer traditional CV risk factors but have a higher mortality rate,” reported Dr. Joseph Timpone, Department of Infectious Diseases, Georgetown University Hospital, Washington, D.C. He indicated that these findings are consistent with current theories that the upregulated inflammatory state in patients with HIV may play a role in the accelerated development of CV disease. The complexity of the role of antiretroviral drugs in suppressing inflammatory cytokines as well as active HIV replication may explain why it has been so difficult to unravel the effects of disease, risk factors, specific antiretroviral therapies, and concomitant diseases, such as renal impairment, on CV risk. Initially, several large cohorts had implicated protease inhibitors (PIs) in elevated CV risk, but it was unclear whether this effect was secondary to elevations in low-density lipoprotein cholesterol, which was common to the first generation agents, or a direct adverse effect on CV function. Subsequently, the NRTI abacavir was also implicated in CV risk in one randomized controlled study and several observational studies, but no mechanism for the increased risk could be identified, and several other sets of data, including another randomized controlled trial, did not corroborate this risk. In new data from the US Food and Drug Administration (FDA), an effort to provide a definitive evaluation also found no excess risk. “We conducted a meta-analysis with 26 randomized controlled trials conducted from 1996 to 2010. Five of these were from ACTG [AIDS Clinical Trials Group], five from academic centers, and 16 from the database maintained by the manufacturer, reported Dr. Kendall Marcus, Washington DC VA Medical Center, Washington, DC, who directed the study on behalf of the FDA. “We were unable to identify any statistically significant association between abacavir use and myocardial infarction [MI].” The analysis included 9832 subjects of which 5028 received abacavir and 4840 did not. The overall risk difference was 0.3%, with a 95% confidence interval (CI) range of -0.24% to 0.30% (See Table 1) (Table 1). Yet, two new studies contribute to the evidence that patients with HIV have an increased risk of MI and other CV events after adjusting for established risk factors. In a study of 81,229 US veterans, the HR was increased by 86% (HR 1.86, 95% CI 1.54 – 2.26) in patients with HIV relative to those without after adjusting for age, ethnicity, hypertension, hyperlipidmaemia, diabetes and smoking. Baseline CD4 counts, HIV RNA levels, and class of antiretroviral drug exposures were not associated with risk after adjustment for risk factors. “Several previous observational studies have associated HIV with an increased risk of MI, but it is essential to account for confounding factors,” reported Dr. Matthew Freiberg, University of Pittsburgh School of Medicine, Pennsylvania. “These data suggest that more intensive efforts at CV risk assessment and modification will be critical to an aging HIV population.”
Neurocognitive Preservation: How Soon to Begin
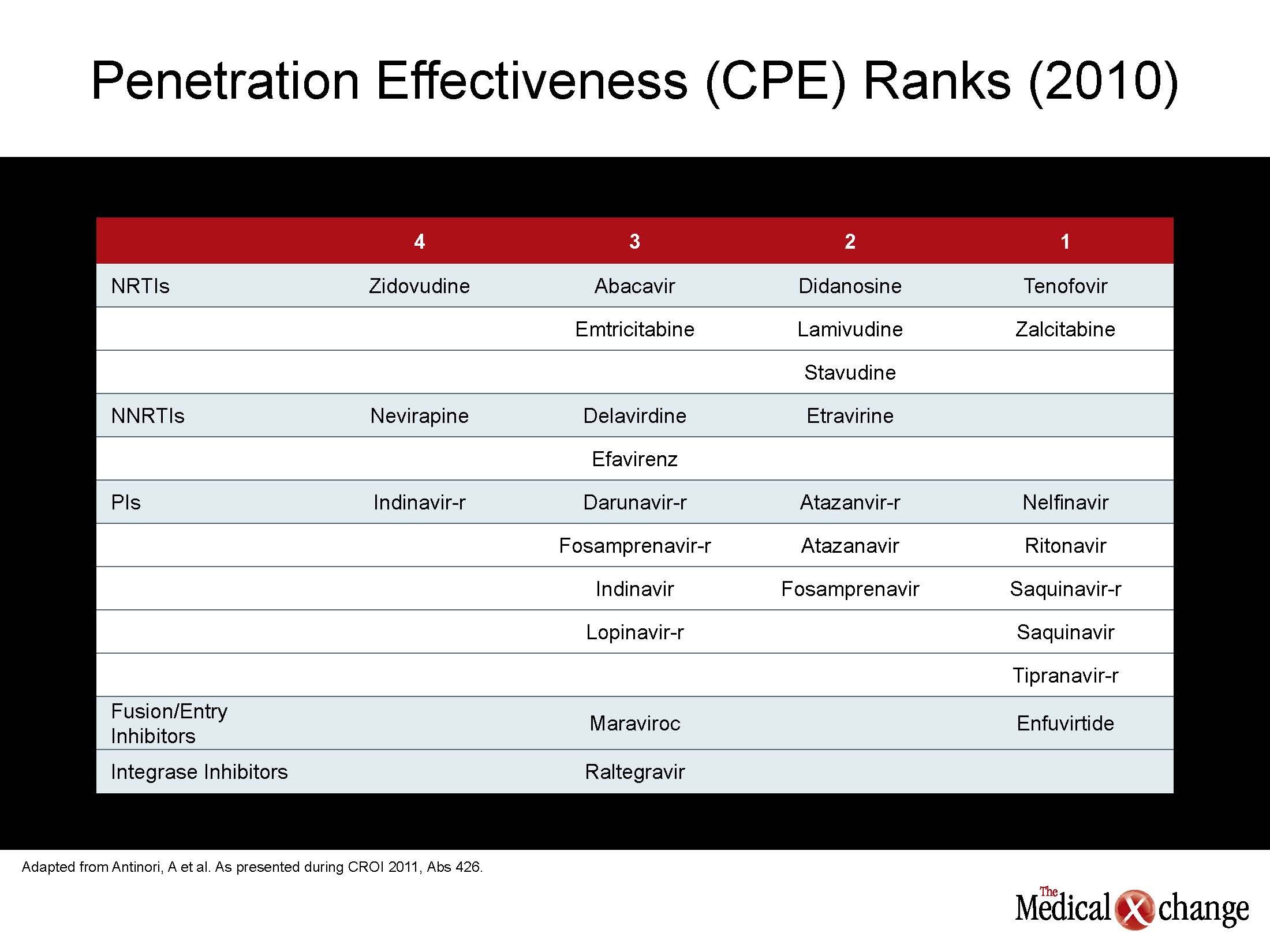
The same conclusion could be drawn about assessing and modifying the risk of neurocognitive decline in patients with HIV. While numerous studies have documented high rates of neurocognitive decline among individuals infected with HIV relative to those without HIV after adjusting for age, new data demonstrates the cerebrospinal fluid (CSF) penetration of antiretroviral agents correlates with lower CSF viral loads and with better neuropsychological performance. In a study presented by Dr. Gabriele Arendt, University Hospital of Dusseldorf, Germany, CSF penetration by a given antiretroviral combination was rated with the 2010 CNS Penetration Effectiveness (CPE) score. The authors found an inverse correlation between CPE and CSF viral loads, so that better penetration produced lower CSF load. A higher CPE score correlated with better performance of cognitive measures (see Table 2) (Table 2).
“The reduction in CSF viral load with better CSF penetration is predictable, but we were also able to see a clinical difference in performance.”
“The reduction in CSF viral load with better CSF penetration is predictable, but we were also able to see a clinical difference in performance. Further studies are needed,” Dr. Arendt reported. These findings were supported by a separate study that also used the 2010 CPE ranking. In this study, plasma samples were drawn from 219 patients with HIV infection. It was found that CPE ranking of 6.0 or greater was highly predictive of undetectable HIV (<50 RNA copies/mL), whereas a ranking below 6.0 was associated with detectable CSF loads. The CPE ranking did not have similar value for predicting plasma viral load. “These findings indicate that antiretroviral combinations with comparable ability to suppress plasma viremia are not interchangeable for controlling viral load in the CSF,” reported Dr. Andrea Antinori, INMI L. Spallanzani, Rome, Italy. “More and longer follow-up data are now needed to see if virus suppression in the CSF reduces neurocognitive complications.” The effort to differentiate antiretroviral therapies for their ability to suppress HIV in the CSF and reduce the risk of dementia is representative of a greater movement toward individualized drug therapy. For many years, the focus on antiretroviral therapy has been on the selection of combinations that are simple and well tolerated. While these attributes remain important, the current data suggest that it may be prudent to individualize therapies based on the risk profile of age-related diseases that appear to be exacerbated by HIV.
Conclusion
Mortality data confirm that patients with HIV in countries where antiretroviral drugs are readily available are more likely to die from non-HIV causes. Evidence that diseases associated with aging, including renal impairment, CV disease, and dementia occur earlier and progress more quickly in individuals with HIV is driving a reorientation in management intended to extend survival. Specific risks, which vary among patients, should be assessed early to allow for judicious intervention, including appropriate selection of antiretroviral therapies.